Your choices so far:
1 Fuel: liquid
| What is your resource? | What do you want to deliver? | What is the service the customer wants? |
| Biomass (digestible sludge) | District cooling | Comfortable indoor climate |
| Biomass (fermentable sludge) | District heating | Electricity |
| Biomass (solid) | Electricity | Process cooling (< 0 °C) |
| Geothermal | Fuel: Gaseous | Process heat/steam (50 - 150 °C) |
| Sunshine | 1 Fuel: Liquid | Process heat (150 - 1000 °C) |
| Water | Fuel: Solid | Process heat (> 1000 °C) |
| Wind | Local cooling (ind. house) | Transport |
| Residual oils/fats etc | Local heating (ind. house) |
Just because a fuel is liquid, it needs neither be good nor clean. Liquid fuels can be produced from biomass in three ways, by the biochemical process known as fermentation, by the thermochemical process known as liquefaction or by transesterification which is a low temperature chemical conversion. The liquid biofuels such as methanol, and ethanol, and biodiesel can, as a rule, be treated as gasoline from safety and fire aspects with some important specific differences.
In conventional ethanol production, starches and sugars are converted into ethanol in a few steps involving a series of enzymes. Subsequently, a biocatalyst is added in the form of yeast that ferments the sugars to ethanol.
The overall and simplified process to convert starch, via glucose, to ethanol and CO2, looks as follows:
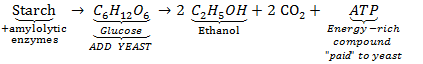
This process is a mature technology that is carried out industrially. When lignocellulose is the substrate for ethanol production, there may be some drawbacks, however. These drawbacks relate to the complexity of the lignocellulosic cell wall components that make up the biomass.
Thus, only some materials, those where fermentable sugars are readily available for the enzymes and the yeast, are naturally suitable for alcohol fermentation while most are not. Todays' production of ethanol is to a significant extent based on dedicated energy crops. This raises the ethical question of large-scale use of agricultural land for energy purposes.
The simplest starting point is to view our planet as a sphere with a circumference of 40000 km. From that assumption, it takes only the simplest math from secondary school to compute the total surface area – which becomes approximately 500 million square km. Now again return to school and remind yourself that approximately 70% of the earths' surface is covered with water – seas and major lakes. Hence the land area is approximately 150 million square km. Now divide this number by the number of people on this planet – about 7 000 million people, and you will find that the land area available to each of us is about 21 000 square meters which is just about 3 football fields. One football field is assumed as 100×70 m2. So three football fields is what every human being has as her "rightful share" of the land of this planet.
Of this land, about 6 000 m2 is infertile like deserts, high mountains and ice-covered etc. Another 6 000 m2 is forested. Almost 6 000 m2 is savannahs, steppes and such meagre land. Of the 3 000 m2 left, 2 000 are rich grassland. Only 1 000 m2 is well suited for agricultural production so that is where to grow food and to grow fibre for clothing.
These are hard facts and cannot be denied. The change of the global climate will affect the distribution and may increase – or decrease – the amount of each biome – but it will not change the size of the planet. Rather, the three football fields are rapidly shrinking because of the population growth.
Imagine making your living only from "your own land" and then make a choice if you want to grow dedicated energy crops on "your" agricultural land – or if you would prefer to grow edible crops. In a sustainable energy system dedicated energy crops are banned unless they are part of a sustainable crop rotational scheme as green fertilizers, nitrogen fixation crops or alike.
This view of the world is the core of sustainable thinking and is of course extremely simplified – but it serves it purpose to illustrate in a simple way what "sustainability" is about: The first step towards a sustainable development is to adopt our use of resources and our use of natural resources to what our planet really has to offer. This puts a strict upper limit to how much biofuel is available. But it also puts focus on the qualities of biomass resources – unless we prefer to compete with agriculture about the 0.15 football fields.
The competing alcohol for energy purposes – methanol – is a common bulk chemical. It is not produced through fermentation but is instead a synthesis product, often starting from methane which may be converted into a syngas (synthesis gas, a gas mixture mainly consisting of CO and H2). The syngas may also be produced via thermal gasification of solid biomass but again, just like for gaseous fuel production from solid biomass, the hot gas cleaning is the crucial step. The synthesis of methanol from syngas is in itself a well-established and fully commercial technique.
Methanol and ethanol are both polar liquids and, hence, slightly acidic and can corrode electropositive metals. Methanol is incompatible with several types of materials normally used in petroleum storage and transfer systems, including aluminium, magnesium, rubberised components, and some other types of gasket and sealing materials. Methanol is also incompatible with with lead, tin, titanium, PVC, polystyrene and Perspex. Ethanol is less corrosive to metals, gaskets, and seals than methanol, but it is still necessary to make sure that any container, transfer lines, and fittings are made from materials that are ethanol-compatible.
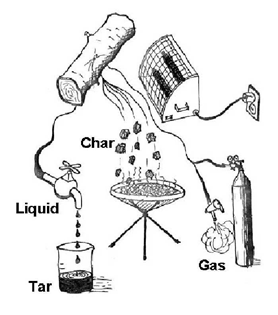
Liquefaction of solid biomass is – like gasification – a thermochemical process but while gasification requires high temperatures, liquefaction is a question of controlling the pyrolysis process. Thermal conversion of solid biomass always begins with drying followed by pyrolysis. Three phases are obtained in pyrolysis a solid residue, char, consisting mainly of carbon and ash, a gas phase consisting of H2, CO, CO2, CH4, C2- hydrocarbons and benzene, and a condensable phase consisting of tar and water.
As illustrated in the schematic, the process requires heat and if the pyrolysis is carried out at about 500 °C and short residence time, about 1 second, the tar yield is maximised.
The liquid phase can also be maximised in direct liquefaction which is performed under high pressure (5-10 MPa cold) and at temperatures around 400 °C. This approach gives a more physically and chemically stable liquid product compared to pyrolysis, thus requiring less upgrading to produce marketable hydrocarbon products.
The complexity of the process in combination with the energy required and the requirements for subsequent post-processing of the bio-oil are hindrances for commercial application of solid biomass liquefaction and hence, solid biomass is not considered as a suitable raw material for liquid fuel production in the present material.
Unlike ethanol and methanol, which are alcohols, biodiesel is an ester that can be made from several types of oils including soybean, rapeseed, sunflower, canola, corn or palm oil, animal fats (tallow), waste vegetable oils (yellow grease), and microalgal oils (lipids). Hence, the resource base includes a large number of industrial and societal waste products from restaurants, food processing industry, agriculture and other sources.
If the oil would be present in a solid material, the first step is extraction, preferably by pressing, a mechanical operation. Once the oil is free from solids follows transesterification. This transesterification is a simple refining process in which the organic oils are combined with alcohol (ethanol or methanol) in the presence of a catalyst, such as sodium hydroxide or potassium hydroxide. The molecules of the raw renewable oil are chemically broken into methyl or ethyl esters of the renewable oil with glycerol as a by-product. The biomass-derived ethyl or methyl esters can be blended with conventional diesel fuel or used as a neat fuel (100% biodiesel).
If the extracted oil is produced from rapeseeds the process produces not only oil but also rapeseed cake, which is suitable for fodder. About 3 tonnes of rapeseed is required per tonne of oil. Rapeseed oil can then be esterified to obtain Rapeseed Methyl Ester (RME) or bio-diesel. This process is already used commercially on a substantial scale, especially in Europe. The next generation will most probably be fatty acid ethyl esters, FAEE, which is a similar product but the alcohol used is mainly ethanol instead of methanol as is the case with FAME.
Neat (100%) biodiesel is incompatible with certain rubbers and plastics, but not with metals. Nitrile rubber and polyurethane-based compounds deteriorate unacceptably by contact with biodiesel while other elastomers such as styrene-butadiene rubber (SBR), butadiene, isoprene, hypalon, silicon, and polysulphide are not resistant to neat biodiesel. Acceptable replacement materials include fluorine-rubber (Viton A) and polypropylene and polyethylene-based plastics.
Therefore, the selection of materials to avoid degradation of seals, fittings, and hoses are important for liquid biofuel applications. It is also necessary to take special precautions to ensure that liquid biofuel is transported or stored in containers and transfer lines that have been specifically selected for that purpose.
Liquid biofuels may certainly be used in CHP- or tri-generation plants but since these can better be using cheaper and less refined fuels that is most unlikely. In the case of small-scale production, they may be used locally for the production of heat and electricity by aid of stationary IC-engines, but again this is not a very good used of these refined, high-grade fuels.
The recommended use is instead as substitute fuels for light fuel oil in industrial processes and as substitutes and complements to gasoline and diesel oil for transportation.