04-03: From Putrescibles to Biogas and Energy
All living organisms need foodstuffs to continue living, or to continue their lives and keep surviving, thriving and in some cases, growing. Proteins, fats and sugars are all of nutritional value to living organisms. All three have a great significance as the donors of building materials and providers of energy for both plants and animals. These are organic molecules, and they can all three be found as constituents of the foods that are derived from both plant and animal matter.
For putrescibles, such as food waste, manure and wastewater sludge, the most suitable methods for energy production are anaerobic digestion and fermentation. This chapter is focused on anaerobic digestion, its processes and properties. Section 04-03-08 presents a short overview of other, less suitable methods for energy production from putrescible.
As mentioned in introduction (01-03), anaerobic digestion takes place in a warmed, sealed airless container (the digester) which creates the ideal conditions for the bacteria to ferment the organic material in oxygen-free conditions. The digestion tank needs to be warmed and mixed thoroughly to create the ideal conditions for the bacteria to convert organic matter into biogas (a mixture of carbon dioxide, methane and small amounts of other gases). Three main types of biogas production (psychrofilic, mesophilic and thermophilic) are described in section 01-03-02.
04-03-01: Fundamental components [1]
Carbohydrates are sugars that the body uses for energy. Simple carbohydrates, also called simple sugars, provide the body with quick energy. They are found in fruit, milk and white sugar. Complex carbohydrates, known as fibre and starch, are broken down in the body slower than simple carbohydrates. They are found in vegetables, bread, rice, oatmeal, whole grains and peas.
Sugars are most often found in nature in foodstuffs, those substances which are ingested. Sugars can be divided into two groups: the simple sugars (monosaccharaides) and more complex sugars (polysaccharides), which are composed of multiple units of monosaccharaides.
Fat: The living body needs fat to grow and to process vitamins. There are many different kinds of fats. Polyunsaturated and monounsaturated fats are good for the body. Such fats are found in nuts and fish, as well as olive, peanut, safflower and canola oil.
Other kinds of fat, including saturated and trans-fat, also called hydrogenated oils, can increase the risk of certain diseases. Saturated and trans-fats are found in butter, fried foods, baked goods, fast food, whole milk and in meat from animals.
Fats are energetically the most concentrated of all sustenance materials. They are found in both plant and animal organisms, serving these living organisms as a source of energy. Thanks to their chemical structure, they can provide the organism with the greatest amount of energy possible from the least amount of matter. Because most animals, including humans, need to keep a store of energy for times when it is needed, it is clear that these stores of fat cannot be shed at any time when an organism deems it so necessary. In other words, an organism cannot rid itself of fat just because it wants to.
• Plant fats are naturally-occurring liquids, with viscous qualities.
• Animal fats are, of course, solids at room temperature.
Protein helps the body grow, builds muscle and give us energy. Protein-rich foods include meats, eggs, avocado, nuts and beans.
Proteins are composed of long chains of amino acids. Amino acids are molecules which are composed of one amino group and one carboxylic group. Proteins can be composed of more than 200 amino acids. Most of these are water soluble. Organic compounds contain a great number of proteins, so that living organisms are constantly, if only gradually, assimilating materials from outside their bodies. Proteins are one of these ingested substances, although some of the substances ingested can be harmful. Among these are the heavy metals, including lead.
Protein and peptide chains can be dissociated, or broken down, into their basic building blocks, or into amino acids. In this reaction, the peptide bonds are broken, and water molecules are bonded onto their constituent parts. This is called a hydrolysis reaction, and it is the opposite of a condensation reaction. The hydrolysis of peptides and proteins takes place in the cells of living organisms, as well as when organisms digest foods in their digestive systems. Enzymes take part in this process as well, accelerating it in a way similar to that of a catalyst.
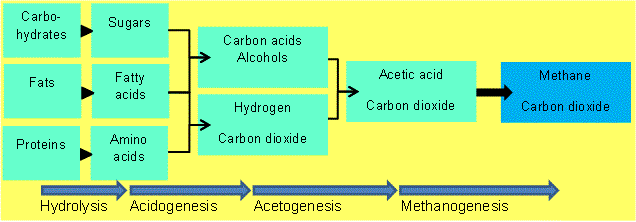 |
| Figure 04-03 1: Main components of feedstock and their conversion processes during anaerobic digestion [2] |
04-03-02: Important factors to make the biomass digestible
When raw material (substrate) is digested in a container, only part of it is actually converted into methane and sludge. Some of it is indigestible to varying degrees, and accumulates in the digester or passes out with the effluent and scum. The "digestibility" and other basic properties of organic matter are usually expressed in the following terms (you may also want to refer to the terminology section in the beginning of the appendix concerning fuel analysis, but be aware that the terminology is slightly different between biochemical and thermochemical applications):
Moisture – The weight of water lost during drying at 105 °C (220 °F) until no more weight is lost.
Total Solids (TS) – The weight of the dry material remaining after drying as above. TS weight is usually equivalent to "dry weight". (However, if you dry your material in the sun, assume that it will still contain around 30 % moisture). TS is composed of digestible organic or "Volatile Solids" (VS), and indigestible residues or "Fixed Solids".
Volatile Solids (VS) – The weight of organic solids burned off when dry material is "ignited" (heated to around 538 °C). This is a handy property of organic matter to know, since VS can be considered as the amount of solids actually converted by the bacteria.
Fixed Solids (FS) – Weight remaining after ignition. This is biologically inert material. As an example, consider the make-up of fresh chicken manure (Figure 04-03 2). Starting from 100 kilograms of fresh chicken manure, 72-80 kilograms of this would be water, and only 15-24 kilograms (75-80 % Volatile Solids of the 20-28 % Total Solids) would be available for actual digestion.
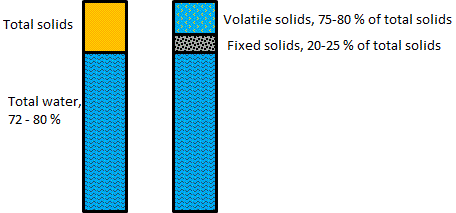 |
| Figure 04-03 2: Properties of chicken manure [3] |
Carbon to Nitrogen Ratio (C/N)
From a biological point of view, digesters can be considered as a culture of bacteria feeding upon and converting organic wastes. The elements carbon (in the form of carbohydrates) and nitrogen (as protein, nitrates, ammonia, etc.) are the chief foods of anaerobic bacteria. Carbon is utilized for energy and the nitrogen for building cell structures.
The bacteria use up carbon about 30 times faster than they use nitrogen. Anaerobic digestion proceeds best when raw material fed to the bacteria contains a certain amount of carbon and nitrogen together. The carbon to nitrogen ratio (C/N) represents the proportion of the two elements. A material with 15 times more carbon than nitrogen would have a C/N ratio of 15 to 1 (written C/N = 15/1, or simply 15).
• A C/N ratio of 30 (C/N = 30/1, 30 times as much carbon as nitrogen) will permit digestion to proceed at an optimum rate, if other conditions are favourable, of course.
• If there is too much carbon (high C/N ratio; 60/1 for example) in the raw wastes, nitrogen will be used up first, with carbon left over. This will make the digester slow down.
• On the other hand, if there is too much nitrogen (low C/N ratio; 30/15 for example, or simply 2), the carbon soon becomes exhausted and fermentation stops. The remaining nitrogen will be lost as ammonia gas (NH3). This loss of nitrogen decreases the fertility of the effluent sludge.
There are many standard tables listing the C/N ratios of various organic materials, but they can be very misleading for at least two reasons:
1. The ratio of carbon to nitrogen measured chemically in the laboratory is often not the same as the ratio of carbon to nitrogen available to the bacteria as food (some of the food could be indigestible to the bacteria; straw, lignin, etc.).
2. The nitrogen or carbon content of even a specific kind of plant or animal waste can vary tremendously according to the age and growing conditions of the plant; and the diet, age, degree of confinement, etc., of the animal.
Nitrogen: Because nitrogen exists in so many chemical forms in nature (ammonia, NH3; nitrates, NO3; proteins, etc.), there are no reliable "quick" tests for measuring the total amount of nitrogen in a given material. One kind of test might measure the organic and ammonia nitrogen (the Kjeldahl test); another might measure the nitrate/nitrite nitrogen, etc. Also, nitrogen can be measured in terms of wet weight, dry weight or volatile solids content of the material; all of which will give different values for the proportion of nitrogen. Finally, the nitrogen content of a specific kind of manure or plant waste can vary, depending on the growing conditions, age, diet, and so forth.
For example, one study reported a field of barley which contained 39 % protein on the 21st day of growth, 12 % protein on the 49th day (bloom stage), and only 4 % protein on the 86th day. You can see how much the protein nitrogen depends on the age of the plant.
The nitrogen content of manure also varies a great deal. Generally, manures consist of faeces, urine and any bedding material (straw, corn stalks, hay, etc.) that may be used in the livestock shelters. Because urine is the animal's way of getting rid of excess nitrogen, the nitrogen content of manures is strongly affected by how much urine is collected with the faeces.
For example, birds naturally excrete faeces and urine in the same load, so that the nitrogen content of chickens, turkeys, ducks, and pigeons are the highest of the animal manures from a nitrogen content point of view.
Next in nitrogen content, because of their varied diets or grazing habits are humans, pigs, sheep, and then horses. Cattle and other ruminants (cud chewers) which rely on bacteria in their gut to digest plant foods, have a low content of manure nitrogen because much of the available nitrogen is used to feed their intestinal bacteria. Even with the same kind of animal there are big differences in the amount of manure nitrogen.
For example, stable manure of horses may have more nitrogen than pasture manure because faeces and urine are excreted and collected in the same small place. Since there are so many variables, and because anaerobic bacteria can use most forms of nitrogen, the available nitrogen content of organic materials can best be generalized and presented as total nitrogen (% of dry weight).
Carbon: Unlike nitrogen, carbon exists in many forms which are not directly useable by bacteria. The most common indigestible form of carbon is lignin, a complex plant compound which makes land plants rigid and decay-resistant. Lignin can enter a digester either directly with the plant material itself or indirectly as bedding or undigested plant food in manure. Thus, a more accurate picture of the C part of the C/N ratio is obtained by considering the "non-lignin" carbon content of plant wastes.
The following table (Table 04-03 1a-c) is a summary of the important chemical properties of organic materials. Values are averages derived from many sources and should be used only for approximation.
| Animal wastes |
Total nitrogen (% dry weight) |
C/N ratio | Manure | Total nitrogen (% dry weight) |
C/N ratio |
| Urine | 16 | 0.8 | Human faeces | 6 | 6 - 10 |
| Blood | 12 | 3.5 | Human urine | 18 | |
| Bone meal | 3.5 | Chicken manure | 6.3 | 15 | |
| Animal tankage | 4.1* | Sheep manure | 3.8 | ||
| Dry fish scraps | 5.1* | Pig manure | 3.8 | ||
| Horse manure | 2.3 | 25* | |||
| Cow manure | 1.7 | 18* | |||
| Table 04-03 1a:Carbon & nitrogen values of wastes Nitrogen is total nitrogen – dry weight Carbon is either total carbon – dry weight – or non-lignin carbon (*), dry weight |
|||||
| Plant wastes |
Total nitrogen (% dry weight) |
C/N ratio | Plant meals |
Total nitrogen (% dry weight) |
C/N ratio |
| Hay, young grass | 4 | 12 | Soybean | 5 | |
| Hay, alfalfa | 2.8 | 17* | Cotton seed | 5* | |
| Hay, blue grass | 2.5 | 19 | Peanut hull | 36* | |
| Seaweed | 1.9 | 19 | |||
| Vegetables** | 2.5 - 4 | 11 - 19 | |||
| Red clover | 1.8 | 27 | |||
| Straw, oat | 1.1 | 48 | |||
| Straw, wheat | 0.5 | 150 | |||
| Sawdust | 0.1 | 200 - 500 | |||
| Table 04-03 1b:Carbon & nitrogen values of plant materials Nitrogen is total nitrogen – dry weight Carbon is either total carbon – dry weight – or non-lignin carbon (*), dry weight. ** Non-legume |
|||||
| Sludge | Total nitrogen (% dry weight) |
C/N ratio | Milorganite | 5.4* | Activated | 5 | 6* | Fresh sewage | 11* |
| Table 04-03 1c: Carbon & nitrogen values of sludge Nitrogen is total nitrogen – dry weight Carbon is either total carbon – dry weight – or non-lignin carbon (*), dry weight |
||
04-03-03: Operating parameters to make the digester work
pH
Methane producing bacteria require a neutral to slightly alkaline environment (pH 6.8 to 8.5) in order to produce methane. Acid forming bacteria grow much faster than methane forming bacteria. If acid-producing bacteria grow too fast, they may produce more acid than the methane forming bacteria can consume. The excess acid will then build up in the system leading to a drop in pH and the system may become unbalanced, inhibiting the activity of the methane forming bacteria. This may stop the methane production entirely.
To prevent this type of failure, the maintenance of a large active quantity of methane producing bacteria is crucial. Hence retained biomass systems are inherently more stable than bacterial growth based systems such as completely mixed and plug flow digesters.
Hydraulic Retention Time (HRT)
Most anaerobic systems are designed to retain the waste for a fixed number of days. The number of days the materials stays in the tank is called the Hydraulic Retention Time or HRT. The Hydraulic Retention Time equals the volume of the tank divided by the daily flow (HRT=V/Q).
The hydraulic retention time is important since it establishes the quantity of time available for bacterial growth and subsequent conversion of the organic material to gas. A direct relationship exists between the hydraulic retention time and the volatile solids converted to gas. Such a relationship for dairy waste is shown in Figure 04-03 3.
Solids Retention Time (SRT)
The Solids Retention Time (SRT) is the most important factor controlling the conversion of solids to gas. It is also the most important factor to maintain digester stability. Although the calculation of the solids retention time is often improperly stated, it is the quantity of solids maintained in the digester divided by the quantity of solids wasted each day.
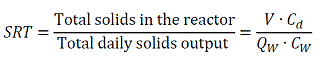 |
Here V is the digester volume; Cd is the solids concentration in the digester; Qw is the volume flow output each day and Cw is the solids concentration in the output flow.
In a conventional completely mixed, or plug flow digester, the HRT equals the SRT. However, in a variety of retained biomass reactors the SRT exceeds the HRT. As a result, the retained biomass digesters can be much smaller while achieving the same solids conversion to gas.
The volatile solids conversion to gas is a function of SRT (Solids Retention Time) rather than HRT. At a low SRT sufficient time is not available for the bacteria to grow and replace the bacteria lost in the effluent. If the rate of bacterial loss exceeds the rate of bacteria growth, "wash-out" occurs. The SRT at which "wash-out" begins to occur is the "critical SRT".
Jewel established that a maximum of 65 % of dairy manure's volatile solids could be converted to gas with long solids retention times. Burke established that 65 to 67 percent of dairy farm manure chemical oxygen demand (COD) could be converted to gas. Long retention times are required for the conversion of cellulose to gas.
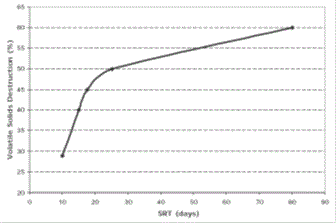 |
| Figure 04-03 3: Dairy farm waste Volatile Solids destruction |
Digester loading (kg/m3/d)
Neither the hydraulic retention time (HRT), nor the solids retention time (SRT) tells the full story of the impact that the influent waste concentration has on the anaerobic digester: one waste may be diluted and the other concentrated.
The concentrated waste will produce more gas per volume unit and affect the digester to a much greater extent than the diluted waste.
A more appropriate measure of the waste on the digester’s size and performance is the loading. The loading can be reported in kilograms of waste (influent concentration multiplied by the influent flow) per cubic meter of digester volume.
A common unit is kilograms of influent waste per cubic meter of digester volume per day (kg/m3/d). One kg/m3/d is equal to 0.0624 lb/ft3/d.
The digester loading can be calculated if the HRT and influent waste concentration are known. | ||
The loading in (kg/m3/d) is simply: |
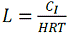 |
where CI is the influx concentration in grams/m3. |
Increasing the loading will reduce the digester size but will also reduce the percentage of volatile solids converted to gas.
Under-loading the process (low substrate input rate) results in low biogas production rate. Although this can prevent process failure, it is uneconomical because the capacity of the process is not fully utilized. Moreover, the process is running at a suboptimal level and the microbial populations are present in a slow and un-dynamic state.
Increasing the load will increase the biogas production but risks overloading, which results in VFA (volatile fatty acids) accumulation. High concentration of VFA decreases pH and makes VFA become more toxic to the methanogens, which may again lead to a process breakdown.
Sufficient nutrients are also important to microbial cell growth. Macro- nutrients such as carbon, hydrogen, nitrogen and oxygen are the main components in biomass cells. Sulphur, phosphorus, potassium, calcium, magnesium and iron are required for specific proteins. These macro-nutrients should be present in the cell, while the micro-nutrients such as nickel, cobalt and copper are required in smaller amounts. Most nutrients can be inhibitory if present in high concentrations. Sulphide and phosphate can decrease the metal ion bioavailability by precipitating. Normally, all the nutrients are present in sufficient quantities in swine and cow manure.
04-03-04: The stability and operation of the biogas process
As follows [4] is reviewed.
The factors affecting the biogas production are mainly the operating conditions and the feedstock.
• Operating conditions such as pH and temperature have a direct influence on the microorganisms.
• Disturbances from the feed include waste composition and concentration as well as toxic and inhibitory compounds. Sometimes, toxic compounds are not initially present in the feed but are produced inside the reactor from substrate degradation (e.g., volatile fatty acids (VFA) and ammonia).
04-03-04a: Parameters that may hinder or slow down digestion
All waste constituents are not equally degraded or converted to gas through anaerobic digestion.
Anaerobic bacteria do not degrade lignin and some other hydrocarbons. The digestion of waste containing high nitrogen and sulphur concentrations can produce toxic concentrations of ammonia and hydrogen sulphide. Wastes that are not particularly water-soluble will breakdown slowly.
For example, dairy wastes have been reported to degrade slower than swine or poultry manure.
The composition of the dairy manure solids is presented in Table 04-03 2. As can be observed from the table, the majority of the volatile solids are composed of cellulose and hemicelluloses. Both are readily converted to methane gas by anaerobic bacteria. However, as pointed out earlier, lignin will not degrade during anaerobic digestion. Since a substantial portion of the volatile solids in dairy waste is lignin, the percentage of cow manure volatile solids that can be converted to gas is lower as compared to other manure and wastes.
The manure characteristics also establish the percentage of carbon dioxide and methane in the biogas produced. Dairy farm waste biogas will typically be composed of 55 to 65% methane and 35 to 45% carbon dioxide. Trace quantities of hydrogen sulphide and nitrogen will also be present.
| Component | Weight-% |
| Volatile (% of total solids) | 83.0 |
| Ether extract (% of total solids) | 2.6 |
| Cellulose (% of total solids) | 31.0 |
| Hemicellulose (% of total solids) | 12.0 |
| Lignin (% of total solids) | 12.2 |
| Starch (% of total solids) | 12.5 |
| Crude protein (% of total solids) | 12.5 |
| Ammonia (% of total solids) | 0.5 |
| Acids (% of total solids) | 0.1 |
| Table 04-03 2: Dairy Manure Composition | |
04-03-04b: Substrate and nutrients
The substrate type and composition will directly determine the biogas yield. The total input of anaerobic substrate is often measured in term of total chemical oxygen demand (COD) or total volatile solid (VS). Distinguishing between the available degradable fraction and the inert fraction is very important in this case, since a considerable fraction of the input COD or VS may be inert (please refer back to text section 04-03-02). Manure, which contains high water and recalcitrant fractions has lower methane yield per VS or COD than easily degradable substrate such as organic waste.
• Lignin is considered as non-degradable in anaerobic reactors.
• Carbohydrates are divided into easily degradable (E) and slowly degradable (S) fractions.
• Lipids, protein and (VFA) are considered as easily degradable.
An example of the different carbon fractions in manure from different sources is shown in figure 03-03 4.
The methane yield of cow manure is in the range of 0.1 – 0.4 m3 CH4/kg VS, while pig manure will exhibit a higher methane yield due to more protein and lipid content, less lignin, and less slowly degradable carbohydrate. This factor strongly depends on the animal feedstock.
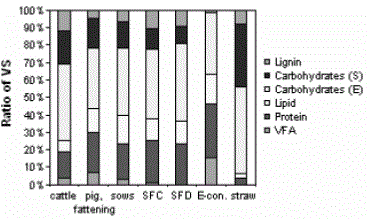 |
| Figure 03-03 4: The average composition of VS in freshly excreted cattle manure, fattening pig manure, sow manure, solid fraction from chemical precipitation of manure (SFC), solid fraction from centrifugation of pig manure (SFD), liquid fraction pre-treated with a decanting centrifuge (E-conc.) and wheat straw [5] |
04-03-05: Biogas properties – Water content
Biogas produced in AD-plants is saturated with water vapour when it leaves the digester. The water may condensate in gas pipelines, even freeze during winter and, together with sulphur oxides and other acidic gas components, cause corrosion. By increasing the pressure or decreasing the temperature, water will condense from the biogas and a dry gas will be produced. Cooling can be achieved naturally by leading it through a pipe in the soil equipped with a condensate trap or with an electric cooler. Water can also be removed by adsorption using SiO2, activated charcoal or molecular sieves. These materials are usually regenerated by heating and/or a decrease in pressure. Other technologies for water removal are absorption in glycol solutions or the use of hygroscopic salts.
Purification systems are now commonly being used to remove the water, siloxanes, VOCs, H2S and carbon dioxide contaminants present in the biogas in a single processing step to produce high purity methane for pipeline sales. Enriching the biogas to pipeline specifications enables the operator to derive substantially greater returns for the biogas since it can achieve its full commercial sales potential. The operator also benefits from the stable demand for natural gas, and avoids the troublesome operating problems with on-site electrical generation.
For the purified biogas to be completely exchangeable to natural gas, the bio-methane will also need a small addition of hydrocarbons such as propane to achieve the correct Wobbe index.
In the Guild process, biogas is compressed to 0.41-0.68 MPa, before being introduced to the Guild adsorption system. The PSA adsorption system removes the water, siloxanes, VOCs, H2S and carbon dioxide to yield a product gas which meets pipeline specifications. Subsequent to the adsorption step, the adsorber vessel is regenerated by reducing the pressure and desorbing the impurities through a vacuum pump, at which point these impurities, and a small portion of the feed methane, leave the system as tail gas. The tail gas can be used as local fuel or flared, as necessary, since it has a relatively low heating value.
04-03-05a: Biogas properties – Chemical composition
The end product of fermentation is a combustible gas mixture, mainly composed as follows:
50 – 75 % methane (CH4);
25 – 45 % carbon dioxide (CO2);
2 – 7 % water (H2O);
< 2 % oxygen (O2);
< 2 % nitrogen (N2);
< 1 % ammonia (NH3);
< 1 % hydrogen sulphide (H2S).
In some cases, biogas contains siloxanes. These siloxanes are formed from the anaerobic decomposition of materials commonly found in soaps and detergents. During combustion of biogas containing siloxanes, silicon is released and can combine with free oxygen or various other elements in the combustion gas. Deposits may be formed containing mostly silica (SiO2) or silicates (SixOy) and can also contain calcium, sulphur, zinc or phosphorus. You may want to refer to the slagging and fouling sections in the appendix about ash properties for more information.
Properties of biogas are pressure and temperature-dependent. They are also affected by the moisture content. The factors of main interest are:
• change in volume as a function of temperature and pressure;
• change in heating value as a function of temperature, pressure and water-vapour content;
and
• change in water-vapour content as a function of temperature and pressure.
The heating value (calorific value) of biogas is about 6 kWh/m3 or 20-22 MJ/m3 and this amount of energy approximately equals that in half a litre of diesel oil. The valuable component, from a fuel point of view, is the methane [6].
The methane potential (refer to text sections 03-03-04b and -04c) is the volume of methane produced during anaerobic degradation in the presence of bacteria of a sample initially introduced, expressed under normal conditions of temperature and pressure (0 °C, 1013 hPa).
As mentioned previously, different substrates will lead to different specific compositions as indicated in table 04-03 3.
The composition of the product gas will also depend on the substrate, of the organic matter concentration and the feeding rate of the digester (see text sections 04-03-03 and 04-03-04).
| Components | Household waste | Wastewater treatment plant sludge | Agricultural wastes | Waste from agri-food industry |
| CH4, % vol | 50 - 60 | 60 - 75 | 60 - 75 | 68 |
| CO2, % vol | 38 - 34 | 33 - 19 | 33 - 19 | 26 |
| N2, % vol | 5 - 0 | 1 - 0 | 1 - 0 | - |
| O2, % vol | 1 - 0 | <0.5 | <0.5 | - |
| H2O, % vol (40 °C) | 6 | 6 | 6 | 6 |
| Total, % vol | 100 | 100 | 100 | 100 |
| H2S, mg/m3 | 100 - 900 | 1 000 - 4 000 | 3 000 - 10 000 | 400 |
| NH3, mg/m3 | - | - | 50 - 100 | - |
| Chlorinated or fluorated organic gases, mg/m3 | 100 - 800 | - | - | - |
| Table 04-03 3: Chemical composition of gas from different substrates. Components in shaded boxes are such components and contaminants that may be problematic. | ||||
The presence of H2S, of CO2 and water makes the raw biogas very corrosive and require the use of adapted materials.
04-03-05b: Biogas properties – Physical characteristics
According to its composition, biogas presents characteristics interesting to compare with natural gas (Table 04-03 4). The gas emerging from the digester is a gas appreciably lighter than air and its heating value is only about half that of natural gas.
| Type of gas: | Biogas 1 Household waste |
Biogas 2 Agrifood industry |
Natural (fossil) gas |
| Composition | 60 % CH4 33 % CO2 1 % N2 0 % O2 6 % H2O |
68 % CH4 26 % CO2 1 % N2 0 % O2 5 % H2O |
97 % CH4 2.2 % C2 0.3 % C3 0.1 % C4 0.4 % N2 |
| HHV, kWh/m3 | 6.6 | 7.5 | 11.3 |
| LHV, kWh/m3 | 6.0 | 6.8 | 10.3 |
| Gas/Air density ratio | 0.93 | 0.85 | 0.57 |
| Density, kg/m3 | 1.21 | 1.11 | 0.73 |
| Wobbe index, kWh/m3 | 6.9 | 8.1 | 14.9 |
| Table 04-03 4: Composition and physical properties of different gases | |||
04-03-06: Biogas upgrading and utilisation [7]
Biogas can be used for all applications designed for natural gas. Not all gas appliances require the same gas standards. There is a considerable difference between the requirements of stationary biogas applications and fuel gas or pipeline quality.
Heating (refer to 04-00-08g)
Boilers do not have a high gas quality requirement. The gas over-pressure usually has to be only around 8 to 25 mbar. It is recommended to reduce the H2S concentrations to values lower than 1 000 ppm which allows to maintain the dew point around 150 °C. In case the H2S concentration is too high and flue gas condensation is applied, the sulphurous acid formed in the condensate may lead to heavy corrosion unless proper materials are used. It is therefore recommended to use stainless steel for the chimneys or condensation burners and high temperature resistant plastic chimneys. Many modern boilers have tin-laminated brass heat exchangers which might corrode even faster than iron chimneys. Where possible, cast iron heat exchangers should be utilised. It is also advised to condense the water vapour in the raw gas prior to the burners.
Removal of water will also remove a large proportion of the H2S, reducing the corrosion and stack gas dew point problems.
CHP-engines (refer to 04-00-08f)
The utilisation of biogas in internal combustion engines is a long established and extremely reliable technology. Thousands of engines are operated on sewage works, landfill sites and biogas installations. The engine sizes range from 45 kW (which corresponds to approx. 12 kWel) on small farms up to several MW on large scale landfill sites.
Gas engines do have similar requirements for the gas quality as boilers, except that the H2S should be lower to guarantee a reasonable operation time of the engine.
Otto engines designed to run on petrol are far more susceptible to hydrogen sulphide than the more robust diesel engines.
For large scale applications (> 60 kWel) diesel engines are therefore standard. Occasionally, organic silica compounds in the gas can create abrasive problems. If so, they should be removed.
Vehicle fuel (refer to 04-00-09a)
The utilisation of biogas as vehicle fuel uses the same engine and vehicle configuration as natural gas. In total there are more than 1 million natural gas vehicles all over the world to demonstrate that gas is a feasible, versatile and commercial vehicle fuel.
However, the gas quality demands are strict. With respect to these demands the raw biogas from a digester or a landfill has to be upgraded. The demands are that the upgrading process delivers a gas which is comparable to natural gas. Compared to the raw biogas, the upgraded gas must
• have a higher heating value in order to reach longer driving distances,
• have a regular/constant gas quality to obtain safe driving,
• not enhance corrosion due to high levels of hydrogen sulphide, ammonia and water,
• not contain mechanically damaging particles,
• not give rise to ice-clogging due to a high water content,
• have a declared and assured quality.
In practice this means that carbon dioxide, hydrogen sulphide, ammonia, particles and water (and sometimes other trace components) have to be removed so that the product gas for vehicle fuel use has a methane content above 95 vol%.
In different countries different quality specifications for vehicle fuel use of biogas and natural gas are applied. Upgraded biogas is actually the cleanest vehicle fuel possible with respect to environment, climate and human health.
For a more detailed technological presentation of biogas upgrading methods, see Biogas upgrading technologies – developments and innovations [8]. For detailed reports on biogas production, purification and use, see IEA Task37 reports [9].
04-03-07: The digestate – quality aspects and use
Most solids not converted into methane settle out in the digester as a liquid sludge. Although varying with the raw materials used and the conditions of digestion, this sludge contains many elements essential to plant life: nitrogen, phosphorus, potassium plus small amounts of metallic salts (trace elements) indispensable for plant growth such as boron, calcium, copper, iron, magnesium, sulphur, zinc, etc.
Nitrogen is considered especially important because of its vital role in plant nutrition and growth. Digested sludge contains nitrogen mainly in the form of ammonium (NH4), whereas nitrogen in aerobic organic wastes (activated sludge, compost) is mostly in oxidized forms (nitrates, nitrites).
Increasing evidence suggests that for many lands and water plants ammonium may be more valuable as a nitrogen source than oxidized nitrogen; in the soil it is much less apt to leach away and more apt to become fixed to exchange particles (clay and humus).
Likewise, important water algae appear to be able to utilize ammonium easier than nitrates. Generally speaking, this is a reversal from the earlier belief by fertilizer scientists that oxidized nitrogen always presented the most available form of nitrogen for plants. Because of this, it has been suggested that liquid digested sludge produces an increase of nitrogen comparable with those of inorganic fertilizers in equivalent amounts.
Most of the previous information showing the poor fertilizer value of sludge was based on municipal sewage sludge. This is a bad measure of the fertilizer value of digested sludge in general. (Municipal treatment flushes away all the fertilizer-rich liquid effluent.) In one case digested sewage sludge was found to contain only about 1/2 the amount of nitrogen in fresh sewage, whereas elsewhere digested pig manure was found to be 1.4 times richer in nitrogen content than the raw pig manure (Table 04-03 5).
Similar results have been found with digested chicken manure. Sludge from digesters can be recycled in a wide variety of ways, both on land and in water and pond cultures. The possibilities are many and only brief descriptions of potentials can be given below.
| Municipal sewage sludge | Nitrogen (% dry weight) |
Sludge from digested manure | Nitrogen (% dry weight) |
| Raw sewage | 1.0 - 3.5 | Hog | 6.1 - 9.1 |
| Digested, 10 municipalities | 1.8 - 3.1 | Chicken | 5.3 - 9.0 |
| Digested, 12 municipalities, Ohio | 0.9 - 3.0 | Cow | 2.7 - 4.9 |
| Digested, 51 samples, 21 cities | 1.8 - 2.3 | ||
| Digested, general average | 2.0 | ||
| Digested, general average | 1.0 - 4.0 | Finished compost | |
| Activated, 5 municipalities | 4.3 - 6.4 | Municipal | 0.4 - 1.6 |
| Activated, general average | 4.0 - 6.0 | Garbage | 0.4 - 4.0 |
| Activated, general average | 4.0 - 7.0 | Garbage | 1.4 - 3.5 |
| Table 04-03 5: Nitrogen Fertilizer Value of Various Sludges and Finished Compost | |||
04-03-07a: The digestate – use in gardening and farming
The application of digested sludge to crops serves a double purpose since it is both a soil conditioner and fertilizer. The sludge humus, besides furnishing plant foods, benefits the soil by increasing the water-holding capacity and improving its structure. Preliminary experiments with garden and house plants have obtained astounding results with the use of sludge from chicken manure digesters. However, there are some things to consider first:
1. Fresh digested sludge, especially from manure, contains high amounts of ammonia, and in this state may act like a chemical fertilizer by force-feeding large amounts of nitrogen into the plant and increasing the accumulation of toxic nitrogen compounds. There is no direct evidence for this, but the possibility exists. For this reason it is probably best to allow the sludge to "age" for a few weeks in an open area (oil drums, plastic pools, etc.), or in a closed container for a few months before using it on crops. The fresher it is the more it should be diluted with water before application.
2. The continued use of digested sludge in any one area tends to make soils acidic. This effect may be counteracted by a little dolomite or limestone added at regular intervals to the sludge-plots, allowing at least 2 weeks interval between applications to avoid excess nitrogen loss. Unfortunately, limestone tends to evaporate ammonia so there may be a temporary nitrogen loss every time the limestone is added to the sludge plots.
3. Unlike digested municipal sludge, sludge from farm wastes does not contain large amounts of heavy metals or salts so there is little danger of applying it too heavily over a period of time. However, one should pay attention to the structure of the soil. If it contains a lot of clay, the sludge will tend to accumulate and possibly present problems in the root area of the plants. In general, keep close tabs on the sludge plots in the beginning until the sludge behaviour in the particular soil has been closely surveyed.
04-03-08: Processes to use the energy
04-03-08a: Suitability for combustion: Suitable in certain conditions
BW and PW often have high moisture content and may include particles of unknown origin, which is why combustion in ordinary combustion equipment is usually not appropriate. Incineration of waste is subject to EU Waste Incineration Directive (2000/76/EC), which places several restrictions if waste is incinerated not in waste incineration plant. If PW is part of municipal solid waste (MSW), then incineration is acceptable in MSW incineration plants. More information about these things can be found in text section 04-04-01.
04-03-08b: Suitability for gasification: Not recommended
The moisture content of the material suitable for thermal gasification has to be less than 25-30%, but BW and PW do not always fulfil this requirement. To prepare waste for gasification it has to be sorted, which raises the price of gas; therefore thermal gasification is only scarcely suitable for waste treatment – see also text section 04-04-02.
04-03-08c: Suitability for pyrolysis / torrefaction: Not recommended
Substrate for pyrolysis and torrefaction should also be preferably dry, moisture content below 30%. Thus unsorted waste will rarely suit for these processes as is also pointed out in text section 04-04-03.
04-03-08d: Suitability for fermentation: Excellent
Usually BW and PW suit well for fermentation because substrate for fermentation has to be preferably liquid, dry matter content of less than 15%. If substrate is drier, water or other liquid can be added.
04-03-08e: Suitability for anaerobic digestion: Excellent
BW and PW usually suit for anaerobic digestion well, because substrate for fermentation has to be preferably liquid, dry matter content of less than 15%. Digestion can be also applied to drier substrate if proper technology (dry digesting technology) and equipment will be used.
References
1 Encyclopaedia about Chemistry and
Factmonster
2 Fry, LJ. Methane Digesters For Fuel Gas and Fertilizer, California, 1973.
3 Dennis A, Burke PE. Dairy Waste Anaerobic Digestion Handbook. Environmental Energy Company, 2001.
4 Kanokwan B. Online monitoring and control of the biogas process. Ph.D. Thesis. May 2006
5 Møller HB, Sommer SG, Ahring BK. Methane productivity of manure, straw and solid fraction of manure. Biomass and Bioenergy. 2004. 26: 485-495.
6 Biogas composition
7 - 9 IEA has a number of reports and publications on biogas
upgrading and utilisation.