04-00: Biomass for energy - Extracting energy
As explained in section 01-00-02a, the energy contained in a biofuel was originally stored by photosynthesis and is latent in the form of energy-rich chemical compounds.
Viewing the photosynthesis qualitatively as
Water + Carbon dioxide + Solar energy ⇒ Energy rich material + Oxygen
one may regard the re-extraction of the bound energy as a reverse photosynthesis:
Fuel + Oxygen ⇒ Water vapour + Carbon dioxide + Heat energy
This is indeed the over-all process but to run this process in an efficient way so as to economize with the natural resources and not produce too much emission on the way, there are some more things that have to be considered.
The first thing is that the heat energy released from the combustion process is, in itself and in most cases, not desired. The aim with the energy extraction process is usually not limited only to produce hot flue gases but to produce either an energy carrier such as electricity, district heating or maybe a fuel aimed for transportation or to actually deliver an energy service of some kind.
Hence it may be relevant first to re-introduce some fundamental terms (EN 14588):
Biomass can be of different origins
Biofuel can be solid, liquid or gaseous and is simply a fuel produced directly or indirectly from biomass
Bioenergy is energy from biomass. Hence, an energy carrier such as electricity produced in a power plant firing bio-methanol may be called bio-electricity.
Energy carriers are commercial products such as biofuel, electricity, steam, district-heating water or alike used to transport energy from a producer to an end-user.
Energy services are what the customer, the end-user, ultimately wants. Energy services can be of many different kinds such as mechanical work (i.e. a moving car or to run an air compressor), thermal energy (i.e. heat for cooking, for climate control or for high temperature processes such as glass melting), light (for illumination or for laser-cutting) or β¦ The energy service desired puts demands on the energy carrier which puts demand on the energy conversion from the fuel to the energy carrier and may, ultimately, put demands on the fuel as such.
What is introduced in this chapter is the process chain from biomass to energy carrier.
04-00-01 Short introduction to processes
The introduction to chapter 02-00 made it clear that biomass is not an infinite resource but must always be handled in such a way as to maximize the total efficiency. The ultimate aim with any energy conversion process is hence to deliver the energy carrier best suited for the end-user requirements with minimal losses throughout the supply chain from raw material (i.e. biomass) to energy carrier.
The ultimate process to release 100 % of the energy is always combustion.
Combustion is β per definition β a complete oxidation of the fuel content of carbon and hydrogen into carbon dioxide (CO2) and water vapour (H2O(g)). During the combustion process, many of the fuel impurities will also oxidise to a higher or lesser extent, some of them, like nitrogen and sulphur, producing harmful emissions (NOx and SOx).
The main heat-releasing reactions are those describing the oxidation of carbon to carbon dioxide and of hydrogen to water vapour and that of water vapour condensing to liquid water:
| C(s) + O2(g) → CO2(g) | qreact = 393.5 kJ/mol at 25 °C and 101 325 Pa (atm. pressure) | |
| C(s) + 1/2 O2(g) → CO(g) | qreact = 110.5 kJ/mol at 25 °C and 101 325 Pa (atm. pressure) | |
| CO(g) + 1/2 O2(g) → CO2(g) | qreact = 283.0 kJ/mol at 25 °C and 101 325 Pa (atm. pressure) | |
| H2(g) + 1/2 O2(g) → H2O(g) | qreact = 241.8 kJ/mol at 25 °C and 101 325 Pa (atm. pressure) | |
| H2O(g) → H2O(l) | qreact = 44.0 kJ/mol at 25 °C and 101 325 Pa (atm. pressure) |
In chemical literature you will find these reaction enthalpies denoted with a negative sign to indicate that heat is released but in this context this is implicit and they are given with positive signs. The above heats of reaction are based on the assumption that the elements (carbon, C, oxygen, O2, and hydrogen, H2), are all present as clean substances is the forms of graphite and pure gas. Since this is not the case with solid fuels one is restricted to use experimental values for the real heat content in the fuel as will be outlined in section 04-00-01a below.
For practical purposes a wet solid fuel consists of two major components, namely:
- Water
- Dry substance
As will be made clear in the next section, the water content is one major factor determining the fuel quality in terms of the energy content.
The water content of the fuel shall be determined according to standard EN 14774 but may be specified in two different ways:
• The water quotient or the moisture quotient is the water content specified on dry basis. Hence if an amount mfuel consists of mw kg of water and mds kg of dry substance, the water quotient is
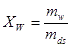 |
or | 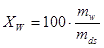 |
% |
• The water content or the moisture content, in contrast, is the water content specified on a wet basis. Hence, for the same example as above, the water content is
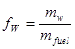 |
or | 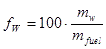 |
% |
It is clear from the above definitions that the water quotient may attain values exceeding 1 or exceeding 100 % while the water content may not.
From a simplified combustion point of view, the dry substance may be regarded as composed from three different substances:
- Ash. This is the residue obtained after complete combustion.
- Char. This is the carbonaceous residue after pyrolysis.
- Volatiles. This is the weight fraction of the dry substance lost during pyrolysis.
The amount of ash in a fuel is normally presented as weight-% on a dry basis and should be determined according to the method devised in EN 14775.
The volatiles content is commonly specified as weight-% on a dry basis and should be determined according to EN 15148.
The standards referred above are those valid for pure solid biofuels β for recovered fuels please refer to EN 15403 and EN 15402 instead. For recovered fuel fractions is may also be of interest to determine the total content of biomass in the material, EN 15440.
As wet, solid fuel enters a hot combustion environment where sufficient amounts of oxygen are present, the process commences through the following steps β schematically:
1 Drying. This is the release of the fuel moisture in the form of vapour and is an endothermal or heat consuming process.
2 Pyrolysis. This is the release of the volatile, partly combustible, matter and is again an endothermal process.
3 Gas combustion. This is the combustion of those components in the volatiles that are combustible. This process is exothermal, i.e. heat is released.
4 Char combustion. This is the final burn-out of the solid residue and is again an exothermal process.
These four steps β often occurring partly or completely in parallel β constitute the complete combustion of the fuel.
For a complete burnout of the fuel, the most crucial parameters are the contact between air and fuel particles, the mixing of air with combustible gases, the residence time and the temperature. Not only mean values are of importance but also the uniformity so that uniform particle size and βproperties as well as temperature uniformity will promote a complete burnout with minimal emissions. This implies that small-scale combustion chambers will be more sensitive to the fuel quality than larger scale. Please also refer to sections 04-00-08c and 04-00-10.
For some applications it may be desirable to separate the combustion process into separate steps. In such cases, the biomass or the solid biofuel is chemically converted into a new fuel such as a liquid or a gas prior to the final combustion. |
Thermochemical conversion makes use of elevated temperature, and in some cases elevated pressure, to convert the solid. For most processes, the heat needed to arrive at the process temperature is generated through a partial combustion of the feedstock. Depending on the actual temperature and on the process layout, more specifically: the heat recovery system, this partial oxidation will introduce energy losses from the feedstock to the product. Also, the temperature of the product fuel will be that of the process. Unless this sensible heat is recovered and used in the process it will represent another loss.
Thermochemical processes introduced here are low-temperature pyrolysis, high-temperature pyrolysis, thermal liquefaction and thermal gasification .
Low-temperature chemical conversion is mainly used to produce diesel substitutes (bio-diesel) from fatty acids. The raw material can be excess vegetable oil from agricultural production, rapeseed oil, soybean oil and alike but also residual cooking oils from β for example β restaurants or from food processing. Basically, the process is to add OH-groups from an alcohol to the molecules. The process thus involves simple bulk chemicals like ethanol or methanol, it runs at low temperatures and the total conversion may be almost 100 % so losses as such are small.
The total energy balance is strongly depending on the addition of alcohol and typically the total energy provided from the alcohol amounts to about 10 β 20 % of the energy contained in the final product. The main by-product is glycerlol, which requires separate handling.
The product is very flexible and can be used as a diesel substitutes as well as to replace light fuel oil in any process.
The only process introduced here is the general production of fame.
Biochemical conversion makes use of micro-organisms to convert the solid material. As pointed out in section
03-00-03 and more detailed in chapter
03-03, micro-organisms thrive at different temperatures, cryophilic (-15 β 15 °C), mesophilic (5 β 50 °C) and thermophilic (50 β 100 °C). The main processes used occur at mesophilic temperatures about 30 β 45 °C and the loss of energy in sensible heat is limited. However, the reason that the micro-organisms are active is that they gain energy from the process, and that energy is taken from the feedstock. So, again, the conversion process introduces an energy loss.
Biochemical processes introduced here are fermentation and anaerobic digestion.
Fuel conversion shall be used only in such cases when the efficiency gain in the final combustion process is big
enough to "pay" for the energy lost in the conversion |
Low-temperature pyrolysis or torrefaction. While a complete pyrolysis requires temperatures in the range of 700 β 900 °C, the process starts already at significantly lower temperatures, about 100 °C. When biomass is heated up β in absence of oxygen β to temperatures about 300 °C, a partial pyrolysis will occur and the material be dried. A pyrolysis at such low temperatures will not fully evaporate the heavier hydrocarbons produced but they will be retained in the dry residue rendering the product some hygroscopic properties. At the same time, the product becomes brittle, its heating value is increased and the density decreases. The main part of the ash will be retained in the solid product and hence the ash content will increase. Some fuel impurities, such as part of the sulphur and chlorine contents, will be released with the pyrolysis gas while others will retain in the solid product.
The heat required for the process represents a loss but can be supplied by combustion of the gaseous and liquid pyrolysis products and may be kept well below 10 % of the total energy contained in the original fuel depending on the moisture content of the feedstock.
The solid fuel thus produced can be used in a variety of processes.
More about solid-solid conversion.
High-temperature pyrolysis or charring. The higher the pyrolysis temperature becomes, the larger the fraction of the volatile components that are released during the process and the smaller the fraction of residual solid. Ultimately, about 70-80 % of the dry substance may be released as pyrolysis products and only about 20-30 % of the dry weight be retained as solid charcoal, so the density decrease is significant. Since practically all hydrocarbons are given off during this process, the product will not have any hygroscopic properties. The high process temperature will release the main part of volatile impurities such as sulphur and chlorine, which will then be present as hydrogen sulphide and hydrochloric acid in the pyrolysis gas.
The heating value of the charcoal, in this case almost pure carbon but with the main part of the mineral ash components still present may be as high as about 30-35 MJ/kg but the total energy used for the pyrolysis process will represent about 10-20 % of the total energy contained in the feedstock β again depending on the original moisture content.
The solid charcoal produced can be used in a variety of processes.
The pyrolysis can also be steered towards a liquid product by setting the temperature to about 500 °C and shortening the residence time. To heat up the feedstock within the short residence time required for maximum output of pyrolysis oil (about 1 second) the process needs a preceding milling of the material.
The total energy balance, including pyrolysis oil and solid residue, is in the same order of magnitude as for high-temperature pyrolysis, i.e. about 80-90 % of the feed energy is retained in the products.
The pyrolysis oil produced will consist of a mixture of hydrocarbons and organic acids, water, volatile fuel impurities and other compounds and will need purification and further upgrading unless it is immediately burned.
More about solid-liquid conversion.
Thermal liquefaction. In case biomass is heated to intermediate temperatures β about 400 °C at pressures around 10 bar β in the presence of steam and carbon monoxide, the formation of a liquid product is maximised. The liquid product quality may β to some extent β be controlled by the use of catalysts. Since the span of molecular weights in the liquid product can thus be limited, the product will generally have a significantly higher quality than pyrolysis oil while it will still contain a significant amount of volatile impurities.
The requirement for a pressurized process will limit the total efficiency to about 80-90 %, in spite of the relatively low process temperature.
The product can be further refined into a high quality fuel.
More about solid-liquid conversion.
Thermal gasification. In thermal gasification, the aim of the process is to convert the solid fuel completely into a combustible gas mixture. To obtain this, high process temperatures (in the range 700 β 1100 °C) are required and a significant fraction of the feedstock energy will be found as sensible heat in the product gas. In the simplest processes (air-blown), the major energy containing components in the product gas will be carbon monoxide, hydrogen and methane and the gas will be heavily diluted by nitrogen. The gas may also be heavily contaminated by heavy hydrocarbons depending on the process layout, co-current, counter current or well mixed. Counter-current processes will yield the lowest tar content in the product gas. Changing medium from air to oxygen or to a combination of oxygen and steam gives the opportunity to significantly change the proportions of the combustible gas components. The high process temperature and the aim to transform 100 % of the solid to gas will also render the main part of the fuel impurities to ultimately enter the gas phase.
Regardless of the gasifier medium remains the fact that a significant amount of the feedstock energy will be present in the form of sensible energy. Thus the total efficiency obtained in thermal gasification is strongly dependent on the system design and on the recovery and use of sensible heat from the product gas.
The choice of gasifier medium and technology are crucial for the usefulness of the product gas β direct combustion or subsequent chemical synthesis to refined products.
More about solid-gas conversion.
Fatty acid methyl esters are mainly used as diesel substitutes (bio-diesel) from fatty acids. The raw material can be excess vegetable oil from agricultural production, rapeseed oil, soybean oil and alike but also residual vegetable oils from β for example β restaurants or from food processing. The feedstock needs to be filtered and free from solid impurities and water prior to the process, so the collection and handling needs be such as to provide a reasonably clean feed. Basically, the process is to add OH-groups to the fatty acid and thus transform it into an ester. This is achieved by the addition of an alcohol, typically ethanol or methanol in the presence of a catalyst, typically at low process temperatures. As compared to the raw vegetable oils, esters have favourable properties with respect to storage (they are more stable). The resulting fuel quality, as measured by the cetane number, is strongly depending on the combination of feedstock and alcohol but some combinations β like coconut oil and ethanol β will typically yield cetane numbers > 70. Such quality fuel, provided it is not contaminated, can serve as a diesel substitute without any need for modifications of the engine while other fuels, such as RME produced from rapeseed oil and methanol (cetane number β 50) may call for engine modifications. It is also important to remember that the final product must be purified so as to be free from residual fatty acids and from water since they are very corrosive. The process involves common bulk chemicals like ethanol or methanol, it runs at low temperatures and the total conversion may be almost 100 %.
The total energy balance is strongly depending on the addition of alcohol and typically the total energy provided from the alcohol amounts to about 10 β 20 % of the energy contained in the final product.
FAME β fatty acid methyl esters β as is the common acronym for this group of fuels, can be used as diesel substitutes as well as diesel additives or can be used to replace light fuel oil in any process.
Fermentation. Fermentation of biomass to alcohol by common yeast is limited by the fact that yeast fungi basically can ferment only such sugars with 6 carbon atoms in them (hexoseβs) while many of the sugars present in biomass are pentoseβs, i.e. they contain 5 carbon atoms. With natural yeast the fermentation is actually limited only to glucose but starch as well as some other types of sugar can be enzymatically transformed into glucose and hence qualify as fermentable. Thus, only some materials, those where fermentable sugars are readily available for the enzymes and the yeast, are naturally suitable for alcohol fermentation while most are not. However, two of the main constituents in plant cell walls β cellulose and hemicellulose β are both basically built up by fermentable sugars. The problem is that they are partly crystalline and are embedded in lignin, so that the sugars are not accessible. Different pre-treatments including dilute acids, hot water or ammonia make it possible to break this structure, decrystallize the cellulose and make the sugars in cellulose and hemicellulose accessible for subsequent fermentation. These processes are still under development and so far they suffer from high costs as well as of some of the by-products being inhibitors to the fermentation process. There is also research on-going to genetically modify micro-organisms so as to render pre-treatment unnecessary.
The product from fermentation is a dilute alcohol that needs be concentrated through distillation to attain fuel quality.
Anaerobic digestion. This is a completely different process from fermentation and one that can work with a wider span of substrates. The main advantage is that this process can make use of feedstock in the form of slurries or sludge such as sewage sludge or industrial fibre sludge, wet manure, waste food and alike, feeds that cannot be used for energy production in any other way. While yeast fungi are the main actors in fermentation digestion takes place through a complex and sequential interaction of β mainly β bacteria. Simplified, the anaerobic digestion starts with the substrate being hydrolysed to soluble organic compounds like fatty acids, sugars and amino groups. These compounds are then further degraded into alcohols and acetic acid, the acetic acid finally being broken down into carbon dioxide and methane. In parallel with the formation of acetic acid there is also a direct formation of hydrogen and carbon dioxide, both of which are partly combined to methane. Since there are thus a large number of intermediate products formed during digestion and since the micro-organisms are very sensitive to their living environment, a thorough process control is needed to avoid accumulation of process inhibitors. Temperature, pH-value, hydrogen content etcetera must all be kept under surveillance to make the process run smooth. The process is also very sensitive to changes in feedstock quality. You will find more details on this in text sections 03-03-03a, b, and c and in 03-03-04a and b.
The product from anaerobic digestion is a mixture of gases, mainly methane about 50 β 70 %, carbon dioxide some 30 β 45 % plus hydrogen sulphide, ammonia, hydrogen chloride and other impurities. The product gas is treated in more detail is 03-03-05a and b.
The gas can be burnt in boilers or in IC-engines without upgrading but most common is an upgrading via pressure-swing adsorption or pressurized scrubbing to methane contents exceeding 95 %.
04-00-01a The heating value
For practical purposes a wet solid fuel consists of two major components, namely:
- Water
- Dry substance
The dry substance, in turn, may be regarded as composed from three different substances:
- Ash. This is the residue obtained after complete combustion.
- Char. This is the carbonaceous residue after pyrolysis.
- Volatiles. This is the weight fraction of the dry substance lost during pyrolysis.
The water content of the fuel shall be determined according to standard EN 14774.
The amount of ash in a fuel is normally presented as weight-fraction on a dry basis and should be determined according to the method devised in EN 14775.
The volatiles content is commonly specified as weight-% on a dry basis and should be determined according to EN 15148.
The energy in the fuel is latent only in parts of the substance and is reduced by the presence of water and inorganic compounds. Since the total energy content is of major importance to the usability of the fuel, the heating value shall now be treated in some detail.
The general method to determine the heating value, or the calorific value, is described in federal standard EN 14918. The scope of this standard is to specify a method for the determination of the gross calorific value of a solid biofuel at constant volume and at the reference temperature 25 Β°C in a bomb calorimeter calibrated by combustion of certified benzoic acid.
The result obtained is the gross calorific value of the analysis sample at constant volume with all the water of the combustion products as liquid water. In practice, biofuels are burned at constant (atmospheric) pressure and the water is either not condensed (removed as vapour with the flue gases) or condensed.
For practical cases, the operative heat of combustion to be used is the net calorific value of the fuel at constant pressure, sometimes referred to as the lower heating value or the effective heating value.
A bomb calorimeter is in principle a well-defined closed container (bomb) completely submerged in a well-stirred and known amount of water. For high accuracy, the vessel containing the water (and hence also the bomb) shall be well insulated.
The theory for the measurement is the following:
• A well-defined amount of fuel is inserted into the bomb and the bomb is then filled up with a sufficient amount of oxygen for a complete combustion of the fuel and also a certain amount of water
• The bomb is then sealed, put into the water bath and is allowed to attain a stable temperature in equilibrium with the water at an initial temperature ti
• The fuel is ignited by the addition of a well-defined amount of energy qign
• As the fuel burns in the closed vessel, the temperature of the vessel and of the surrounding water will successively increase and shall be recorded
• Once the temperature stabilizes at a final temperature tf, this temperature is documented and the total change in temperature is used to evaluate the total change of sensible energy in the system
Care must be taken that the temperature interval during the measurement [ti , tf] includes 25 °C and is not too small.
After subtraction of the ignition energy and any other known additions, the resulting amount of energy represents the gross calorific value of the sample at constant volume. This value is then re-calculated to finally represent the operational value, namely the net calorific value of the sample at constant pressure. The calorimeter shall be calibrated prior to each measurement to determine the instrument constant.
The gross heating value or the higher heating value includes not only the chemical energy released from the combustible substance but also the heat released during condensation of the water vapour after combustion, i.e. 44.01 kJ/mol for the reaction H2O(g) β H2O(l).
The total amount of water present in the combustion gases is determined by the sample moisture content and by the amount of water formed during the combustion of hydrogen inherent in the combustible substance and the result is also affected by the fuel content of sulphur and some other elements. Hence, to do the re-calculation, the ultimate analysis of the combustible substance must be known. The relevant standards are EN 15104 and EN 15289.
The net heating value, qnet, or the lower heating value at constant pressure is the most relevant measure to use for practical purposes. This value represents the total energy that can be released from the fuel when it is completely burnt and when the combustion products, the flue gases including water vapour, leave the combustion chamber in gaseous form. Since, thus, the net heating value does not contain the condensation heat for water vapour the value becomes lower than the gross heating value.
In the normal case, the laboratory determination of net heating value will not always be representative for the fuel actually received and the value must be re-calculated to represent the real fuel at the energy plant. The federal standard to be applied in this case is EN 15296.
As shown in section 03-00-02c, the order of magnitude for the net heating values for dry, ash free biomass are in the range 10β25 MJ/kg. If the net heating value q°NET is known (MJ/kg) from a laboratory analysis for a specific fuel sample with moisture content f°W and ash content f°ASH, expressed as weight fractions on a wet basis (f°W) and on a dry basis (f°ASH) respectively, then a reference value qNET,DAF can be computed from
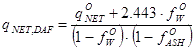 |
MJ/kgDAF |
For indicative values please refer to table 03-00 1 in section 03-00-02c of this handbook.
As long as the fuel delivered can be considered to consist of the same dry and ash-free substance, i.e. as long as the fuel originates from the same biological specie, the net heating value at any moisture content fW or ash content fASH, again expressed as weight fractions on wet basis (fW) and on dry basis (fASH) as they are usually measured, can now be calculated from the reference value by:
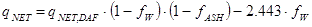 |
MJ/kg |
| Enter data from laboratory protocol: | |
| Net heating value as received (qoNET), MJ/kg: | |
| Water content as received, % by weight: | |
| Ash content, % by weight in dry substance: | |
| Reference heating value (qNET,DAF), MJ/kg | |
An example:
Suppose the laboratory protocol says that a fuel sample had moisture content (wet basis) 31.6 % and ash content
(dry basis) 4.3 %. The net heating value, the lower heating value, for the sample "as received" at the laboratory (i.e. 31.6 % water, 4.3 % ash) was 12.47 MJ/kg.
With these values, one may now compute the reference value for this biomass:
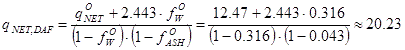 |
MJ/kgDAF |
For future deliveries of similar biomass β i.e. biomass from the same biological specie β one then needs to know only the water content and the ash content to calculate the heating value.
Assume that one delivery of the above biomass with qNET,DAF=20.23 MJ/kg has ash content (dry basis) 6.35 % and water content 48.72 %.
The net heating value with this delivery is then (MJ/kg real fuel as received):
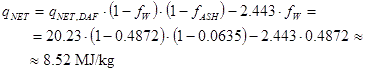 |
For waste fractions, this becomes more complicated since they are composed of different raw materials and cannot be assumed to always represent the same dry and ash-free substance. This is dealt with in more detail in chapter 04-04.
04-00-02 Solid biomass ⇒ Solid biofuel ⇒ Energy
When formed through photosynthesis biomass is solid. Thus, the most obvious way to make use of the energy latent in the biomass is by direct combustion processes. This is also the shortest possible route with the highest potential to avoid losses and to maximize the total efficiency. The pre-treatment of the biomass into biofuel is also the simplest possible, in many cases involving only open-air drying (section 03-00-02c) and fragmentation (03-00-02d).
The energy released from direct combustion will be in the form of hot combustion products or flue gases and the process to make use of this heat thus involves heat transfer from the hot gases to the desired product.
In case of high-temperature industrial processes such as glass-making, steel-making, cement production or alike, impurities or chemical compounds in the flue gases may have an adverse effect on the fuel quality. Is such cases, precaution must be taken to protect the product from the gases and the combustion equipment may become too complex to be viable. For some processes, however, this is not a major problem and cement kilns as well as calcining kilns are frequently fired with sorted waste fractions as a complementary fuel. For these applications, the fuel must be dry enough to provide the desired temperature. Throughout history, the main process to arrive at extremely high temperatures has been charing.
For the production of process steam β for chemical industry or for power production alike β impurities are of less importance unless the flue gases contain too much corrosive components. With virgin biomass this is usually not a problem while sorted and clean waste fractions may prove problematic. For waste fractions, the material in superheaters should be chosen in accordance and with due consideration of the fuel composition. The temperatures desired for steam production are usually well below 700 °C and the demands on fuel dryness are hence not crucial.
For hot-water production aimed for space heating, in single buildings as well as in district-heating applications, the temperature restriction is evens less and so are the restrictions posed by fuel impurities.
| Biomass | Pre-treatment | Biofuel | Final process/-use |
| Woody biomass felling residues trimmings etc |
Comminution & drying | Wood-logs Chunks Chips |
Direct combustion: Heat production Steam raising |
| Woody residues saw dust shavings etc |
Comminution, drying, milling & compaction |
Wood-briquettes wood-pellets high quality |
Direct combustion: Heat production Steam raising |
| Woody residues saw dust shavings etc |
Comminution, drying & compaction |
Wood-briquettes wood-pellets low quality |
Milling & pulverized-fuel combustion: Heat or steam production, maybe co-firing |
| Field residues including energy crops |
Comminution, drying | Shreds and strands | Direct combustion: Heat production Steam raising |
| Field residues including energy crops |
Comminution, drying, milling, mixing with binder (lignin?) & compaction |
Agro-briquettes agro-pellets high quality |
Direct combustion: Heat production Steam raising |
| Field residues including energy crops |
Comminution, drying, milling, & compaction |
Agro-briquettes agro-pellets low quality |
Milling & pulverized-fuel combustion: Heat or steam production, maybe co-firing |
| Solid municipal waste clean fraction only |
Comminution | Shreds and strands | Direct combustion: Heat production Steam raising |
| Solid municipal waste clean fraction only |
Comminution, drying, milling, mixing with binder (lignin?) & compaction |
MSW-briquettes MSW-pellets high quality |
Direct combustion: Heat production Steam raising |
| Solid municipal waste clean fraction only |
Comminution, drying, milling, & compaction |
MSW-briquettes MSW-pellets low quality |
Milling & pulverized-fuel combustion: Heat or steam production, maybe co-firing |
| Industrial & societal waste demolition wood etc |
Comminution | Chunks or chips | Direct combustion: Heat production Steam raising |
| Various | Charring or torrefaction | Charcoal | Milling & pulverized-fuel combustion: Heat or steam production in co-firing |
Table 04-00 1: Common routes for solid biomass ⇒ solid biofuel ⇒ energy
Out of 11 listed process chains in the table, four ends up in pulverized fuel combustion preceded by a milling of the solid fuel while the remaining seven are considered best suited for direct combustion of the fuel as it is.
Mechanically durable pellets or briquettes, i.e. high-quality products, are well suited for use in single-family houses, the characteristics of which are outlined in section 04-00-08. Relevant standards for pellet and briquette quality are EN 15210-1 and 15210-2 respectively but also EN 14961-1 through 14961-3 may be relevant in some cases.
For the use of chunks, chips, shreds and strands, the scale must not be too small unless combustion and environmental performance shall be sacrificed and district heating (section 04-00-08e) may be the most appropriate. Relevant fuel quality standards are EN14961-4 and -5. Firewood and wood chunks may also be used in single-family houses and for these cases you are referred to text sections 04-00-08a through 04-00-08d.
Pulverized fuel combustion is commonly used in large-scale coal fired plants and solid biofuel suitable for milling, such as non-durable pellets and briquettes, are well-suited to be used as a complementary fuel in such plants. This is outlined in text section 04-00-08m.
04-00-03 Solid biomass ⇒ Liquid biofuel ⇒ Energy
For some applications it may be desirable not to use the biofuel in solid form but to convert it. One major sector demanding a converted fuel is the transport sector. The main reason for this is that the infrastructure already established for the transport sector is built for the distribution of high energy density fuels in liquid form, i.e. gasoline and diesel oil. High energy density liquid fuels are also advantageous in many process industry applications because of the simplicity in handling and the high efficiencies attained.
So there may be different reasons for solid fuel conversion; handling economy or process economy and efficiency being the foremost. The third aspect is the potential gain in environmental performance: a separation of fuel impurities may sometimes be included in the fuel conversion process. Hence, a contaminated raw material may be converted into a clean fuel or the combustion conditions may be improved so as to minimize the emissions from the final combustion process where the fuel is actually used.
The ultimate aim for a liquefaction process is to retain all the energy in the feedstock but to transform the energy carrier completely into a liquid. The total energy contained in the liquid product may partly be sensible heat, depending on process temperature, and partly chemically bound energy.
Since any conversion will involve energy losses, it is important that these losses are "paid for" either by savings in the handling step, by environmental benefits or by an efficiency increase in the final use of the fuel.
The energy input necessary for the fuel conversion process should as far as possible be supplied from a refuse fuel fraction. Sometimes, though, like in the case of thermal liquefaction, the process demands that the fuel is fragmented into small particles and the process demands an elevated pressure. In such cases, external energy β usually electricity β will have to be brought into the process and the cost and the total energy balance will be affected accordingly.
Biochemical processes, such as fermentation or digestion, that run at low temperatures, may exhibit a better total energy balance than thermal processes but there will always be a loss. The biochemical processes demand that the feedstock is not too dry but ongoing research work aims at lowering the demands on water content. Hence, these processes are mentioned here though they will mainly be treated in sections 04-00-06 and -07.
To actually improve the properties of the fuel it is important to remember that the conversion process must involve the separation of one or more components. Pyrolysis of a moist, solid and contaminated, material followed by a subsequent combustion of the wet oil and the char will not per se increase the combustion temperature or reduce the amounts of emissions.
Only if the water is removed from the oil will the combustion temperature increase but then this will involve a further process step and requires more external energy input.
The same thing applies to fuel impurities: only if the impurities are removed will the environmental impact be reduced and this will involve external energy input.
Hence, fuel conversion must not be mistaken for fuel improvement: improvement may or may not be included in the conversion process.
| Biomass | Pre-treatment | Biofuel | Final process/-use |
| Woody biomass, field residues or solid municipal waste, too moist for combustion |
Comminution and drying followed by autothermal pyrolysis or liquefaction vapour removal from gas |
Pyrolysis gas, oil and tar Charcoal |
Direct combustion: Heat production Steam raising |
| Sugar-rich residues from agriculture or agro-industry including energy crops |
Comminution followed by fermentation and distillation | Bio-ethanol | Transport fuel: Gasoline additive or Direct combustion |
| Cellulose-rich residues from agriculture or forestry including some waste fractions |
Enzymatic/acidic cellulose degradation followed by fermentation & distillation | Bio-ethanol | Transport fuel: Gasoline additive or Direct combustion |
| Oil-rich residues from agriculture or agro-industry including energy crops |
Cold pressing followed by esterification | Bio-oil or bio-diesel | Transport fuel: Diesel substitute or Direct combustion |
| Various | Thermal gasification to synthesis gas quality gas cleaning from catalyst poisons followed by chemical synthesis |
Bio-methanol, bio-diesel, bio-DME etc | Transport fuel: Diesel substitute Gasoline additive or substitute or Direct combustion as a light fuel-oil substitute |
Table 04-00 2: Routes for solid biomass ⇒ liquid biofuel ⇒ energy
As seen from the table, the main market for the liquid fuel produced is the transport sector, the main alternative being direct combustion. For industrial use of pyrolysis oil as a substitute for fossil fuel oil, the quality of the pyrolysis oil is crucial. Not to contain corrosive components, not to be hazardous and at the same time to be stable enough to fulfil the requirements set by storage and handling, the pyrolysis oil will typically require an advanced upgrading before being delivered.
04-00-04 Solid biomass ⇒ Gaseous biofuel ⇒ Energy
Thermal gasification is one of the most complicated conversion processes but it is also one of those that open up the most possibilities for subsequent processing.
The ultimate aim for a gasification process is to retain all the energy in the feedstock but to transform the energy carrier completely into gaseous form. The total energy contained in the product gas will partly be sensible heat due to the elevated temperature and partly chemically bound energy. To achieve this, all the volatile components must be released from the solid material and all the solid char must be gasified.
The process involves a huge number of endothermal (energy demanding) chemical reactions such as drying, devolatilisation and the formation of methane from carbon monoxide and water vapour and to run these energy-demanding reactions there will be a need for energy to be supplied to the process. To achieve this and render the total process autothermal (i.e. no external energy is needed to arrive at the process temperature desired), part of the fuel needs to be combusted. To minimize the fraction of fuel lost through this partial combustion the feed needs to be reasonably dry, typically less than 20 % moisture.
The main advantages gained by thermal gasification of solid biomass are four:
• Since the temperatures during gasification are lower than during direct combustion, feeds involving ashes with low melting points can be treated with less operational problems. This group includes a large number of herbaceous feeds and waste fractions. Hence the process may be more flexible with respect to feed properties than direct combustion.
• The specific volume of fuel gas (m3/kgfeed) produced during gasification is significantly less than the total volume of flue gas (m3/kgfeed) produced in direct combustion. This may facilitate the economic removal of gaseous fuel impurities prior to the final combustion of the gas. However, gas cleaning usually requires the gas to be cooled and hence will involve a loss of sensible energy from the gas.
• The product gas quality can be set within wide limits, from the simplest gasifiers that produce wet, nitrogen- and tar laden gas mainly suitable for direct combustion to highly advanced gasification processes producing synthesis gas well aimed for subsequent chemical upgrading to a number of products. To improve the fuel flexibility in an energy plant a simple gasifier directly connected to a gas-fired steam boiler may be sufficient while a synthesis-gas quality process connected to a Fischer-Tropsch reactor may be the basis in β for example β a bio-DME production plant. The final product needs not be limited to fuel but this process concept can be the heart of a so-called biorefinery with almost any number and variety of products.
• If connected to a combined-cycle power production process the total efficiency as calculated from latent energy in the biomass feed to electricity delivered to the grid may be improved. To be viable, this application will require a large-scale power plant.
The drawbacks are mainly the economy β the investment and the operational cost for the gasifier does not replace investment and operational cost for the combustion plant, it comes on top. The second drawback is the losses and the fundamental fact that losses tend to be inversely proportional to scale. Hence, to keep losses low, the scale must be large.
To actually improve the properties of the fuel it is important to remember that the conversion process must involve the separation of one or more components. Gasification of a moist, solid and contaminated, material followed by a subsequent combustion of the gas will not per se increase the combustion temperature or reduce the amounts of emissions.
Only if the water vapour is removed from the hot gas will the combustion temperature increase but then this will involve a cooling process and requires that the sensible energy and the condensation energy are recovered.
The same thing applies to fuel impurities: only if the impurities are removed will the total environmental impact be reduced and this may involve external energy input.
Hence, gasification must not be mistaken for fuel improvement: improvement may or may not be included in the conversion process.
| Biomass | Pre-treatment | Biofuel | Final process/-use |
| Various, dry enough for autothermal process |
Autothermal gasification | Product gas: Air-blown: Low heating-value gas Oxygen-blown: Medium-high heating-value gas Oxygen-and-steam-blown: Synthesis gas |
Low heating-value gas: Direct combustion Medium heating-value: Direct combustion Synthesis gas: Chemical synthesis to methane, DME, hydrogen ... |
Table 04-00 3: Routes for solid biomass ⇒ gaseous biofuel ⇒ energy
04-00-05 Liquid biomass ⇒ Liquid biofuel ⇒ Energy
The main result from photosynthesis is solid biomass. However, some parts of plants such as seeds and nuts may contain extremely energy rich organic oils that are or can be extracted and used. Examples are sunflower seeds, linseed, olives and a number of other utility crops. Such oils are also extensively used for cooking and the sources for liquid biomass are hence not only agricultural process industry but also restaurants and β to some extent β households.
Though such oils may be used as fuel without any preparation at all, they are notoriously unstable and also contain compounds that are easily oxidizable or corrosive. To be of practical use in an energy context these products need be stabilized so as to be able to be stored for an extended time without changing their properties significantly and this usually involves a transformation where the mixture of fatty acids present in the feed is transformed to a mixture of esters. The process was introduced above in section 04-00-01. The fuel thus produced, depending on the feedstock, may be marketed and used as a diesel oil substitute aimed for the transport sector and may also be used to replace light fuel oil in most any industrial process.
The process involves the addition of alcohol and this will limit the over-all energy economy of the process.
| Biomass | Pre-treatment | Biofuel | Final process/-use |
| Vegetable oils from agriculture or from food industry, liquid waste oils as spent cooking oil or alike | Filtering, water separation followed by esterification | Fatty acid methyl esters (FAME) | Transport fuel: Diesel substitute or additive or Direct combustion as a light fuel-oil substitute |
Table 04-00 4: Routes for biomass sludges ⇒ liquid biofuel ⇒ energy
In this case, the transformation process aims at actually improving the fuel with respect to its storage properties and to produce a fuel that is more uniform with respect to its properties than the feedstock. To obtain the improvement in quality a preparatory step is necessary to remove water and impurities and external energy input is required in the form of alcohol.
04-00-06 Biomass sludge ⇒ Liquid biofuel ⇒ Energy
Already in text section 01-00-03, it was pointed out that the choice of feed for a biomass-based energy system must be based on thorough environmental and sustainability considerations. Questions discussed in that section included
• Will the use of biomass for energy threaten commercial use for other purposes?
• Will the use of biomass for energy threaten the supply of food?
• Will the use of biomass for energy threaten the biological diversity?
• Will the use of biomass for fuel increase the total atmospheric emissions?
For sludge β biomass residues suspended in water with water contents often exceeding 90 % - the competitive uses are limited and the re-cycling of nutrients from the sludge to be used as fertilizers can be simplified.
Sludge is produced in a number of industrial processes, fibre sludge from pulp and paper industry being one example, sugar-rich sludge from sugar production being another, and is also produced in society, sewage sludge being an obvious example.
The wet sludge lends itself excellent for biochemical conversion, the water content is so high that the heating value (see text section 04-00-01a) is negative and its ash content as expressed as weight fraction of the dry substance is often very high. Hence it cannot β without drying (see text section 03-00-02c) β be used in any thermochemical processes. You will also want to refer to text section 03-03-02 about this.
To produce a diesel substitute, i.e. a suitable mixture of high molecular weight oils or esters, a complicated process involving chemical synthesis would be required and the most common routes instead aim at alcohol production through fermentation.
Provided the cleanness and the quality of the feed is thoroughly controlled the wash can be used as a fertilizer or even β but this will require an extremely advanced quality control β for fodder.
| Biomass | Pre-treatment | Biofuel | Final process/-use |
| Cellulose-rich residues from agriculture or forestry including some waste fractions |
Enzymatic cellulose degradation followed by anaerobic digestion and gas upgrading |
Bio-methane | Direct combustion: Heat production Steam raising Transport fuel |
| Sugar-rich sludge mainly from agro-industry | Fermentation, distillation | Bio-alcohol | Transport fuel: Gasoline additive orDirect combustion |
Table 04-00 5: Routes for biomass sludge ⇒ liquid biofuel ⇒ energy
04-00-07 Biomass sludge ⇒ Gaseous biofuel ⇒ Energy
While fermentation (see text sections 04-00-01 and 04-00-06) is limited to such sludge where the content of fermentable sugars is high enough, anaerobic digestion can be applied to a broader spectrum of substrates. However, lignin, cellulose and fatty acids are all difficult to digest and for some sludge a pre-treatment involving lignin/cellulose degradation or a thermal degradation of the fatty acids (at about 70-80 oC) may be advisable.
| Biomass | Pre-treatment | Biofuel | Final process/-use |
| Lignin-lean biomass, agricultural or industrial residues and wet sludges including sewage sludge and manure |
Comminution of large solids followed by anaerobic digestion | Raw biogas | Direct combustion: Heat production Steam raising |
| Lignin-lean biomass, agricultural or industrial residues and wet sludges including sewage sludge and manure |
Comminution followed by anaerobic digestion and gas upgrading |
Bio-methane | Direct combustion: Heat production Steam raising Transport fuel |
| Cellulose-rich residues from agriculture or agro-industry incl. some waste fractions |
Enzymatic/acidic cellulose degradation, anaerobic digestion, gas upgrading | Bio-methane | Direct combustion: Heat production Steam raising Transport fuel |
Table 04-00 6: Routes for liquid biomass ⇒ liquid biofuel ⇒ energy
The total yield from anaerobic digesters depend strongly on the quality of the feedstock, on the temperature, pH-value and on the retention time.
Anaerobic digestion may be applied in a number of scales extending from small scale at single farms up to large installations in conjunction with wastewater treatment plants, see also chapter 03-03.
04-00-08 Processes using direct combustion
Biomass is a limited resource (see the introduction to chapter 02-00) and any use of biomass for energy purposes shall always strive for the technology that provides the best system efficiency. This is not necessarily the same as using the most advanced technology but in some cases it can be advisable to introduce bioenergy by low-tech solutions to get things going and suspend the introduction of high-tech processes until the basic concept has been firmly established. This may for example include the replacement of gas-, coke- or oil-fired boilers for space heating with pellet stoves or βboilers, it may include the installation of very primitive anaerobic digesters for the production of single farm-house cooking gas and alike.
The simplest processes do often require only a direct combustion of the solid fuel for the production of comfort heat, tap water and β eventually β electricity. These processes will now be discussed along an increasing scale from the single-family house up to large district heating systems.
04-00-08a: Single-family houses
The energy supply to a cluster of buildings may be planned according to any of two completely different strategies:
• Fuel distribution in combination with a de-centralized (i.e. individual) energy production.
• Centralized energy production in combination with energy distribution.
The first option requires that the individual house is equipped with its own boiler or stove and this means that each unit becomes small (typically less than 25 kW thermal power). The main fuel alternatives in this small scale, become gas, kerosene, wood logs and wood pellets. The small scale at each unit will effectively prohibit cooling production or CHP so the individual units become "pure" heat producers aimed to provide the house with comfort heat and tap water and they will have no effect on the electricity balance. The good thing with this system solution is that β as long as the fuel supply works β it is robust. The bad things are that this system cannot be the basis for electricity production, it cannot be the basis for cooling production and it will not contain any advanced environmental control.
The characteristic for single-family houses is that the variations in energy need may be very different from hour to hour. For example will the fact that one person in the household takes a shower suddenly demand a rise in heat supply to the hot water system and if windows are opened for intensive ventilation the demand for heating suddenly increases radically. Also the opposite is true: A party with 20 participants will suddenly increase the heat supply to the house with about 2-3 kW (one person releases approximately 100 W only in the form of body heat and if they are dancing the heat release increases) and there might suddenly be a demand for cooling.
04-00-08b Single-family houses - the heating system
Central heating in single-family houses can be water-borne β which is preferred in case of biofuel β or it may be air-borne. In case of water-borne heating, the production of tap water is normally integrated in the same boiler but β of course β with a separate heat exchanger coil. In case of air-borne heating, the production of tap water becomes a completely separate system.
With water-borne systems, the boiler may be complemented with an accumulator tank to even out thermal load. The accumulator tank shall be large enough to cover the average heat demand of the house, tap water and heat combined, during 24 hours with a temperature drop of about 20 °C. With an accumulator tank integrated in the system it is simple to integrate solar heating with the system by a separate heat exchanger coil in the accumulator. There are also some domestic boilers that have extra-large water volumes β though not as large as can be obtained by a separate accumulator β and also comprising solar heating connections.
Since the water volume in the boiler itself β or in the combined boiler and accumulator β is significant, a water-borne system will provide a thermal inertia that simplifies the control of the system. A sudden increase of the hot water demand, for example, can then be supplied from the stored energy in the system so that the demand can be met while the heat input (for example a pellet burner) might need some time to get started. The larger the thermal inertia, the longer start-up times may be accepted.
With air-borne heating systems, the thermal inertia is close to zero and the heating system must respond almost instantaneously. For this kind of systems, air-heating, pellet fired stoves are available on the market. For the supply of hot water, though, separate water heating devices become necessary.
A central heating system may represent a significant investment for the single household and it is important that the equipment bought fulfils all reasonable quality criteria. The European federal standard EN 303-5 covers testing procedures and marking of boilers for this sector. Furthermore, EN 15316-4-7 covers the dimensioning methods for single-house heating systems.
04-00-08c Single-family houses - the combustion system
Any fireplace β be it in a boiler or a stove β is designed to work best with a specific fuel. Good combustion equipment β fed with the correct fuel at the correct rate β will provide a complete burnout of the fuel in combination with minimal amounts of air pollutants. This is especially important in case of individual house heating with individual boilers. A cluster of buildings β all emitting for example high concentrations of soot or heavy hydrocarbons β may well cause the outdoor air quality to become a local health hazard. Also may nitrogen oxides be formed, and they, too, represent a source of air pollution.
As said in text section 04-00-01, combustion proceeds through four steps β schematically:
1. Drying. This is the release of the fuel moisture in the form of vapour and is an endothermal or heat consuming process.
2. Pyrolysis. This is the release of the volatile, partly combustible, matter and is again an endothermal process.
3. Gas combustion. This is the combustion of those components in the volatiles that are combustible. This process is exothermal, i.e. heat is released.
4. Char combustion. This is the final burn-out of the solid residue and is again an exothermal process.
These four steps β often occurring partly or completely in parallel β constitute the complete combustion of the fuel. This will pose problems in the case of chunk-wood or wood-log firing.
Old domestic boilers for wood firing β up until the 1960βs β were typically equipped with a cast-iron combustion chamber completely submerged in the water to be heated.
With such a construction, the walls of the combustion chamber were cold, with a temperature close to that of the water. Hydrocarbons and tar released from the wood logs will then β if entering into these cold wall zones β either condense to form small droplets or at the very least will the flames be extinguished so that unburned hydrocarbons will escape from the combustion chamber. This temperature non-uniformity is even more pronounced by the fact that the wood-logs are large as compared to the physical dimensions of the fireplace interior.
In modern designs, the combustion chamber is lined with ceramic insulation, reducing but not eliminating the problem of non-uniformity.
The most modern wood-log boilers are designed for downwards combustion. In these, the gases from the coldest log will pass down, through the bed of already burning and glowing material, so that the gas is maintained at a high temperature throughout combustion, leading to even lower emissions of hydrocarbons. These boilers are also designed for batch firing, so that a specific load of logs are input, ignited and allowed to burn out completely. Hence a modern boiler save a lot of manual work since it does not require a continuous feed as did the old boilers.
Opposed to the irregular firewood or chunk wood, pellets will have a diameter of 6, 8, 10 or 12 mm, a particle density for the individual pellet exceeding 1100 kg/m3 and they will be mechanically robust. They will also have a low moisture content, typically about 12 %, corresponding to an energy content (heating value) about 16-18 MJ/kg or almost 5 kWh of thermal energy per kg. (to obtain kWh from MJ, divide by 3.6) So pellets are small as compared to the fireplace interior and they are uniform in properties.
Since wood pellets also have a smooth surface, a uniform shape and relatively constant properties as a whole, they lend themselves well to small-scale automatic feeding and firing systems.
Pellet firing systems for domestic use in single-family houses generally fall into two different categories, namely
• Burners to be mounted in hot-water boilers
• Self-contained stoves for air heating, containing combustion chamber, burner and day storage tank assembled. For some units the day tank might be separate while quite often all the parts are assembled in one physical unit
Both types of system solutions are commercially available as off-the-shelf units. The reliability with the commercial systems is high and the need for maintenance is low.
The household market for wood pellets is rapidly expanding and pellets may be bought in bags as well as in bulk in many places over Europe.
Installing a wood pellet system in a single family house with a central heating system includes installing a boiler with a pellet burner and a pellet store. With an air-borne heating system the installation consists of a pellet stove with a store.
For the boiler, it is important that the fireplace is large enough to contain the flames from the pellet burner. Typically, the flames from wood pellet combustion are larger than those from oil or gas firing and the fireplace thus needs be bigger. In case the fireplace is too small, the flames will be extinguished close to the walls, resulting in hydrocarbon and soot emissions as explained above.
In some cases, a boiler originally aimed for oil firing can be used also with pellet burners but in most cases it is recommendable to acquire a boiler designed for pellets. Such boilers will also be designed to accommodate a reasonably large amount of ash, thus prolonging the intervals for ash removal. Finally, designated pellet boilers will also be designed to simplify the ash removal.
In case a pellet system replaces old oil- or gas fired systems it is crucial to check the status of the chimney. Pellet firing will result in larger flue gas volumes and the chimney must be able to cope with that. In case a pellet burner is mounted in an old boiler the flue gas temperatures may also become higher.
Provided the pellet quality, i.e. the mechanical durability of the pellets, is high enough, most crucial for the functionality is the burner ignition process and this holds true for air-heating stoves as well as for burners in hot-water boilers. A pellet burner, be it mounted in a stove or installed in a boiler, is designed to bun pellets. If the pellets break during handling and feeding so that the burner is effectively fed with saw-dust, the burner will not work properly.
Provided the pellet feed works and provided the burner is actually running, it is very scarce that the flame goes out. Instead, stoppages are almost exclusively caused by the burner failing to ignite.
04-00-08d Single-family houses - planning aspects
The energy system in single-family houses cannot take very high investments and is usually controlled using a simple on-off system. With water-borne systems the control parameter is normally the water temperature in the boiler or the accumulator while air-borne systems, especially if the system is distributed with several stoves and heat sources, may have thermostats in several rooms. With water-borne systems the temperatures in individual rooms are set by aid of radiator thermostats.
The control system may also include a timer control so that the temperature set points are different between night and day, thus providing a lower night temperature. The theoretical basis for such a control is that a lower indoor temperature overnight will contribute to a lower mean temperature: 20 °C during 18 hours plus 16 °C during 6 hours yields a temperature average of 19 °C per 24 hours period.
The actual effect of a low temperature set point overnight depends strongly on the total thermal inertia of the building; the larger the thermal inertia β the lower the effect. Hence this will be an alternative in light, wood structure and wood frame, buildings, while heavy stone buildings will show no or only marginal energy savings by this method.
With water-borne systems, the boiler may be complemented with an accumulator tank to even out thermal load. The accumulator tank shall be large enough to cover the average heat demand of the house, tap water and heat combined, during 24 hours with a temperature drop of about 20 °C. With an accumulator tank integrated in the system it is also simple to integrate solar heating with the system by a separate heat exchanger coil in the accumulator. There are also some domestic boilers that have extra-large water volumes β though not as large as can be obtained by a separate accumulator β and also comprising solar heating connections.
Since the water volume in the boiler itself β or in the combined boiler and accumulator β is significant, a water-borne system will provide a thermal inertia that simplifies the control of the system. A sudden increase of the hot water demand, for example, can then be supplied from the stored energy in the system so that the demand can be met while the heat input (for example a pellet burner) might need some time to get started. The larger the thermal inertia, the longer start-up times may be accepted.
With air-borne heating systems, the thermal inertia is close to zero and the heating system must respond almost instantaneously. For this kind of systems, air-heating, pellet fired stoves are available on the market. For the supply of hot water, though, separate water heating devices become necessary. The water heaters must β in combination with air-borne heating β have extremely short response times and gas heaters or electric heaters would be preferred.
In case cooling is needed in a single-family house, this is usually arranged by installing separate AC-units in the rooms where the need is most pronounced, or to have a mobile AC-unit that is moved to the room where it is needed. For single houses only compressor cooling machines are applicable.
Hence, the stationary energy system in single houses can have only two roles, namely to provide heat and to provide tap water.
04-00-08e District heating
The energy supply to a cluster of buildings may be planned according to any of two completely different strategies:
• Fuel distribution in combination with a de-centralized (i.e. individual) energy production.
• Centralized energy production in combination with energy distribution.
The first option, covered above in text sections 04-00-08a and more specifically 04-00-08c, requires that the individual house is equipped with its own boiler or stove. The bad things with this solution are that such a system solution cannot be the basis for electricity production, it cannot be the basis for cooling production and it will not contain any advanced environmental control.
The second option β using centralised energy production and then distributing the energy to a large number of customers β has the advantage that the production unit becomes larger and hence may accommodate not only more advance environmental control but also a more advanced process, depending on the scale. There are no strict limits for the scales but from a qualitative point of view one may distinguish three general categories, the minimum demand being that there are at the very least two separate buildings involved so that there is a meaning to the word "distribution":
Small systems. In the current context, a system will be considered small if the thermal load for a full year is too small to accommodate steam production. This type of systems might for example provide the heat needed for tap water and comfort heating in a university campus, for a number of public buildings in a cluster or for the buildings in a hospital area.
Intermediate systems. In the intermediate scale, the system may incorporate steam production. There can be several reasons for this: There may be a process industry or a laboratory needing steam among the customers, or the system may simply be big enough to be able to carry the extra cost associated with a steam boiler.
Large systems. A large system is one large enough to make power (i.e. electricity) production a main option. In this case, the main focus is often switched from heat sales to electricity sales but it is a mistake to look away from the heat market.
The different scales will not only affect the types of products but will also open up for different types of fuel qualities. Any fireplace is designed to work best with a specific fuel. Good combustion equipment β fed with the correct fuel at the correct rate β will provide a complete burnout of the fuel in combination with minimal amounts of air pollutants.
As said in text section 04-00-01, combustion proceeds through four steps β schematically:
1. Drying. This is the release of the fuel moisture in the form of vapour and is an endothermal or heat consuming process.
2. Pyrolysis. This is the release of the volatile, partly combustible, matter and is again an endothermal process.
3. Gas combustion. This is the combustion of those components in the volatiles that are combustible. This process is exothermal, i.e. heat is released.
4. Char combustion. This is the final burn-out of the solid residue and is again an exothermal process.
As was also pointed out in text section 04-00-01, the relative size of the fuel particles to the fireplace volume, the temperature difference between burning gases and cold walls and a number of other parameters, will all affect the burnout ratio and the environmental performance of the combustion unit. Environmental aspects will be more treated in text section 04-00-10. As the physical size of the fireplace itself is increased the combustion process as a whole becomes more robust and tolerant to fuel quality variations. This is also connected with the fact that larger combustion units will normally be equipped with combustion control systems while small units only have the most rudimentary control.
Of course, there is a limit to this but as a very general rule-of-thumb one may say that while a single-family heating system (≤ 0.1 MWth) requires high-quality pellets or briquettes for a good environmental performance, a 1 MWth boiler can be fed with wet stem-wood chips or uniform residues from a process industry. Similarly, a 5 MWth boiler can be fed with wet felling residuals or agricultural field residues. At a scale about 10 MWth one can even feed the boiler with wet MSW (N.B. a clean fraction without heavy metals or other inorganic and hazardous contaminants) and still maintain fully acceptable environmental performance.
Obviously, there are grey zones and the above numbers are only indicative and strongly depending on the design of the boilers but they may still serve as orders of magnitude for the combination of fuel resources and combustion technology.
04-00-08f: District heating and CHP - electricity production
In case the district heating system is large enough to carry the investment and operational cost for a more advanced process, it may be viable to install a steam turbine for electricity production.
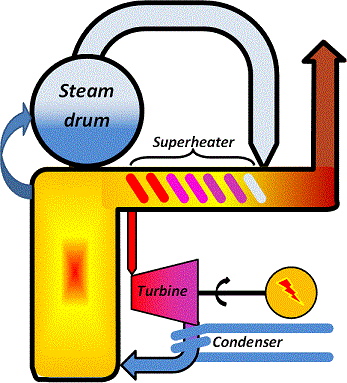
The over-all principle of electricity production using a steam cycle in condensing power-plants, the most common concept, is illustrated in the schematic. You will notice that there is no district heat production in this concept but only electricity production.
 |
 |
| Figure 04-00 1: A generic condensing power production plant | |
The function is that
1. Water is heated at a high pressure in the boiler walls.
2. The water is then transferred to the steam drum where it evaporates to form steam.
3. Steam is taken from the drum and heated to high temperature in the superheater.
4. The hot, high-pressure steam runs through the turbine to rotate it.
5. The turbine shaft is connected to a generator to produce electricity.
6. To maximize steam throughflow, a vacuum is maintained at the turbine outlet by condensing the steam using a cooling medium at the lowest possible temperature.
7. The condensate is the returned to the boiler walls for another cycle.
What is shown in the schematic is the simplest possible process outline. In real applications this will look much more complicated, but the principle will still be the same.
Since the turbine will provide a resistance to the steam flow, it is obvious that the higher the total pressure difference across the turbine, the more steam will flow through it. In modern fossil gas fired steam-cycle power stations, the steam temperature at the turbine inlet is approaching 600 °C and the inlet steam pressure is up to 160 bar. Limiting for the steam temperature and steam pressure are material constraints and there is strive to raise these values further.
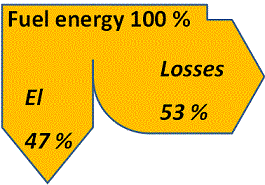
In case the fuel contains impurities to make the flue gases corrosive the steam temperature and pressure will be limited by the superheater stress caused by the combination of a high internal pressure and corrosion attacks from the outside. Hence, for coal- or biofuel-fired power stations the steam temperature and pressure are usually limited to about 550 °C and 140 bar respectively. This puts an upper limit to the total fraction of the fuel energy that can be converted to electricity and values for the electricity efficiency scarcely exceeds 47 % in coal- or biofuel-fired power plants based on steam cycles. The main part of the remaining 53 % of the energy supplied through the fuel is found as low-temperature heat losses via the cooling medium supplied to the condenser, the remainder being lost through flue gases and transmission.
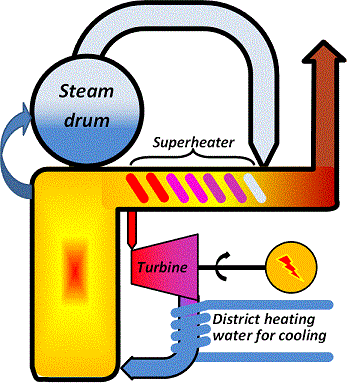
Combined heat and power production β CHP
To make use of the energy loss to the cooling medium the temperature after the turbine must be allowed to attain higher values, so that the cooling medium becomes hot enough to be useful. The price paid for this is then that the vacuum at the turbine outlet is reduced, the steam throughflow is reduced and the electricity efficiency drops to about 40 % or slightly less.
The gain is that the energy that would be lost in case of condensing production now becomes a valuable product and can be sold to district heating customers. The schematic below illustrates the concept.
 |
 |
| Figure 04-00 2: A generic CHP plant and its over-all performance | |
Feeding a steam-cycle condensing power plant with 100 % fuel energy thus approximately yields up to 47 % electrical energy and 53 % losses
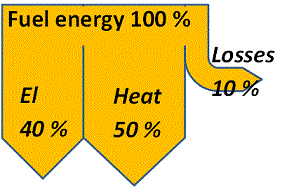
With a CHP-plant the distribution instead becomes approximately 40 % electric energy, 50 % heat energy and 10 % losses.
Combined heat and power production allows for a flexibility in product mix. Assume that the unavoidable losses, as illustrated in the figure, are 10 % and that the remaining energy can, at the most, be converted to 40 % electricity thus leaving 50 % for heat. But the 90 % need not be divided 40+50 as in the schematic. It could just as well be split 20+70 or 10+80 or 0+90 or any other distribution depending on the demand.
IC engines
For some applications in small scale and when a gaseous or liquid fuel is available β such as landfill gas or biogas from a farm-size digester β one may also use an internal combustion engine (IC-engine). In this case, a generator is directly connected to the engine shaft and the cooling water is used for heating. Care must be taken about the corrosive properties of the gas and a gas cleaning, for example a simple scrubbing, is advisable. For such systems, the electricity efficiency may be in the span 15-30 % and the total efficiency about 50-80 %, strongly depending on gas quality and on the engine.
Aside from steam turbines, the second major method to produce electricity is by gas turbines.
Gas turbines
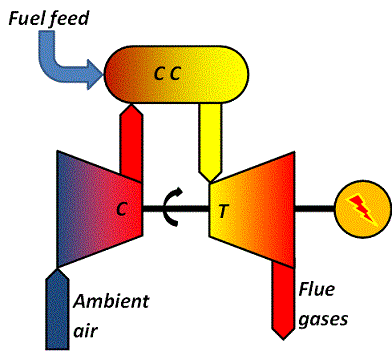
Gas turbines are different from steam turbines in that they contain an integrated, pressurized combustion chamber and a compressor, just like the jet engines in aeroplanes. The over-all principle is illustrated in the schematic.
 |
|
| Figure 04-00 3: A generic gas-turbine plant | |
The turbine "T" is mounted on a common shaft with a compressor "C" and a generator.
Once the unit is running, ambient air is brought into the compressor, compressed to some 15-25 bar and released into a pressurized combustion chamber "C C".
Into the combustion chamber is also added fuel.
The hot (about 1100 °C) and pressurized flue gases are then allowed to expand through the turbine.
The gases leaving the turbine will still be hot, about 400-600 °C.
In a gas turbine process, the fuel energy shall not only supply the turbine and the generator but also the compressor. Hence, the total efficiency becomes relatively low and values are often less than 40 %.
For CHP applications, the hot flue gases are used to produce district heating. Opposed to the case of the steam cycle, where district heat production reduced the attainable electricity efficiency, this is not the case with gas turbines.
Turbines are very sensitive to deposition on and to erosion of the turbine blades and in the case of a gas turbine the blades are directly exposed to the flue gases from combustion. The fuel must therefore be free from ash and from such impurities that can form corrosive gases. Solid biomass, as well as sorted waste fractions or indeed any solid fuel are thus effectively excluded as gas-turbine fuels while clean converted fuels such as bio-methane, bioethanol and alike can well be used.
The combined-cycle concept in CHP application
In sufficiently large power stations, gas turbines and steam turbines are combined to "combined cycles". A combined cycle power production process makes use of the hot flue gases from the gas turbine to produce steam, uses the steam in a steam turbine and may finally produce district heating from the steam turbine outlet. The general schematic illustrates the process. You will be able to identify the gas turbine cycle and the steam turbine cycle.
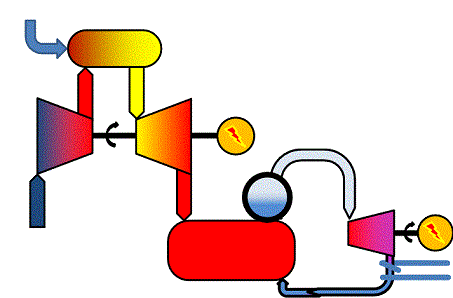 |
|
| Figure 04-00 4: A generic combined-cycle plant | |
Assuming the gas turbine in a combined cycle application has the total electricity efficiency ηGT and the steam turbine has the electricity efficiency ηST, the total electricity efficiency of the combined cycle plant, ηCC, will be
ηCC = ηGT + ηST - ηGT*ηST
So if the gas turbine has an efficiency at 38 % (ηGT = 0.38) and the steam turbine 45 % (ηST = 0.45), the total electricity efficiency becomes almost 66 %. Values like this are indeed obtained in modern, fossil-gas-fired large scale combined-cycle power plants when running without district heat production, i.e. in condensing mode.
The same plant, when also delivering district heating, may be assumed to have gas turbine efficiency still at 38 % (ηGT = 0.38) but the steam turbine efficiency will be lowered to maybe 38 %, too (ηST = 0.38). The total electricity efficiency then drops to about 62 %. This is still far beyond anything that can be reached using only steam turbines or only gas turbines.
BIGCC
The potential for high electricity efficiencies is the background for the so-called Biomass Integrated Gasification and Combined Cycle (BIGCC) concept. Though the gasification process in itself will induce energy losses and though the gas cleaning necessary to protect the gas turbine will also induce energy losses, the whole process may still be advantageous from an electricity efficiency point of view. However, to be feasible, this is only viable in large scale and has, so far (2011), not been demonstrated in commercial applications.
04-00-08g District heating and CHP - the system
For small scale systems, without steam or electricity production as defined above, the over-all system will be described by the following schematic. Please be observant that there may well be several boilers and β of course β lots of final customers but the diagram is as simplified as it may be.
 |
| Figure 04-00 5: A generic district heating system with no electricity production |
Thus, the system consists basically of three serial circuits, a boiler circuit, a distribution circuit and any number of customer circuits. In practice, each customer will have two customer circuits β one for the tap water and one for the comfort heating system water. These will be manifest as two separate coils in the customer heat exchanger but for simplicity only one is included in the schematic.
The main components and their roles
The boiler-end heat exchanger
Looking at the schematic above it is obvious that the district heating system contains two heat exchangers β one at the boiler end and one with the customer. From a purely theoretical point of view, it might seem wise to exclude the boiler-end heat exchanger and to use the boiler circuit water also for the distribution, but this is not recommended for two reasons:
1) The part in the system most exposed to and most vulnerable to leakages is the distribution network, which may well extent for several kilometres across the township. In case of a major leakage β and if the boiler water is also the circulating water β the boiler will be dry and may suffer severe damage. Hence, the heat exchanger protects the boiler.
2) For maximum efficiency in the system as a whole it is important that the return water in the distribution system is as cold as possible β preferably below 50 °C. Also the forward water should have the lowest possible temperature, and during summer this may be as low as about 65 β 70 °C. Bringing water of such low temperature into (indirect) contact with the flue gases in a heat exchanger may induce condensation of water in the flue gases with subsequent corrosion in the heat exchanger. This becomes more pronounced the higher the water content in the fuel. Hence the heat exchanger adds to the versatility and the robustness of the system.
The main circulation pump
A critical component in the system is the distribution pump, the pump distributing the hot water to the customers in the system. For robustness, there should always be installed a spare pump.
The power consumption in the main pump will β roughly β be proportional to the square of the velocity of the water in the pipes. Hence, doubling the velocity of the water will approximately quadruple the power consumption. On the other hand: If the velocity in the pipes is doubled, then the residence time of the water in the tubes is halved and hence the temperature loss during the time the water travels through the pipes is reduced.
Several decades of experience shows that a reasonable compromise between pumping power and heat loss is reached at water velocities (average for the whole system) about 1 β 5 m/s. The capacity of the main circulation pump and the diameters of the distribution pipes are thus chosen so that the water velocity ends up in this interval and the thermal power delivered is primarily adjusted by aid of the forward temperature and not by adjusting the flow.
The piping
The long-term economy and reliability of a district heating system is mainly determined by two parameters, namely the total system efficiency and the life-time. Both these are to a great extent determined by the quality of the piping.
It is obvious that if the water velocity is set, then a small district heating system will mainly consist of pipes with a small diameter and the ratio of pipe surface area to water flow becomes large. Hence the heat losses through the pipe walls also become relatively large.
While large-scale district heating systems may exhibit pipe losses in the range of 5 % or even less in some cases, the small-scale networks will inherently have higher losses, up to 15, maybe even 30 %, in some installations. This cannot be avoided but the reason that district heating is still advantageous in small scale is because it provides means for fuel flexibility and for efficient flue-gas cleaning due to its scale.
Installing a district heating system in a domestic area, it will replace maybe 100 or more individual-home boilers which are typically not fuel flexible but dependant on one single β maybe fossil β fuel and which will have no flue gas cleaning at all. Replacing these with a modern, biofuel-fired boiler with flue-gas cleaning is in most cases an environmental improvement β even if the system efficiency may be as low as 70-80 %, energy wise.
Replacing the individual boilers with compact heat exchangers, which are clean and silent devices and also removing the burden of chimney-sweeping from the individual households are side effects that are β according to questionnaires from Sweden β are also appreciated by the customers. To this comes the reduction of the risk for fires in the individual houses.
Nevertheless; the losses in the system may be significant and it is important that the pipes first installed are of the highest attainable quality with respect to their thermal insulation. It is also advantageous to use double tubing where the forward water pipe and the return water pipe are both enclosed by a common insulation with only one outer mantle.
The second demand put onto the distribution pipes β on top of the demand for thermal insulation β is the demand that they are diffusion sealed. One major reason for failures in district heating networks is corrosion, and corrosion is mainly caused by diffusion of oxygen into the distribution water. There are two ways to prevent the presence of oxygen gas dissolved in the water: Seal the tube walls so that oxygen cannot enter and β second β bind the oxygen if it enters. Both measures should be taken because corrosion may completely destroy the system quite rapidly.
In order to avoid in-diffusion of oxygen into the system, metallic distribution tubes should be chosen in the first instance. Even the highest quality plastic materials are still today (2011) inferior with respect to gas diffusion and since the network will contain metallic components β heat exchangers, valves, pumps etc β corrosion will be a problem once oxygen gas enters the distribution water circuit. New materials are continuously being developed and most probably will the heavy metal tubes successively be outflanked by light polymers, but so far are the metal tubes (often copper) the superior.
The second measure to abate the internal corrosion problem is to bind any oxygen entering into the system. So far this has often been done using hydrazine (N2H4) but since the hydrazine is in itself weakly toxic and may cause allergenic reactions, the use of this substance is debatable. It should also be mentioned that there are indications that hydrazine may be carcinogenic. Hydrazine is still used in Swedish district heating networks β though of course in very low concentrations β but is has been forbidden is some of the European states.
The customer heat exchanger
The temperatures and the flow rates through the customer heat exchanger will determine the temperature of the water with the customer. For the tap water circuit, the temperature at the customer side should not be allowed to fall below about 55 °C to avoid the establishment of legionella bacteria.
For the comfort heating water, though, the temperature level is determined by the need and by the heating system actually used by the customer. With a water-borne floor heating system the temperature needed is only about 30 °C while old radiator systems may require temperatures up to 70 or even 80 °C to provide a comfortable indoor climate.
The customer heat exchangers must thus be adjustable within fairly wide limits with respect to the customer side water temperature at the same time as they should be cheap. Care must therefore be taken during the procurement phase for the heat exchangers so that all these demands are met.
04-00-08h: District heating and CHP - solid fuel
As pointed out in a previous section (04-00-08e), there is good reason to classify different systems into three categories:
Small systems. In the current context, a system will be considered small if the thermal load for a full year is too small to accommodate steam production.
Intermediate systems. In the intermediate scale, the system may incorporate steam production.
Large systems. A large system is one large enough to make power (i.e. electricity) production a viable option.
Most systems installed are in the small-medium range, roughly up to 10-15 MW thermal input, and these would with few exceptions be too small to host electricity production.
The vast majority of installations in these scales will be equipped with moving-grate boilers aimed for hot-water production or for steam production. The main reason for this is the robust construction, well aimed for most any type of solid fuel. Moving-grate boilers are used for waste incineration as well as for pellet combustion, in straw-fired boilers and in a number of other applications. The construction can cope with low-melting ash and with high-ash contents alike. There is also a multitude of manufacturers to choose from in a number of countries which makes it possible to choose a supplier with experience in the fuel at hand.
As was emphasized in chapter 03-00 (text section 03-00-04) boilers are designed for different moisture contents in the fuel. The moisture content will be determining the temperatures attained inside the combustion chamber. The temperature level is set by cooling the combustion chamber β dry fuel requires more intense cooling, wet fuel may not need any cooling at all. The cooling is, in turn, set by the thickness of the lining in the combustion chamber and the aim is to meet the approximate target temperatures illustrated in the schematic below, showing the main features of a moving-grate boiler.
 |
| Figure 04-00 6: A generic moving-grate boiler |
Solid fuel is pushed, by a pusher or a screw, onto a grate made up from bars arranged like a flight of stairs. The actual construction of the grate varies between manufacturers, in some designs it is horizontal, in some designs it is made out of rollers etc. But to illustrate the function the description will be restricted to only one design.
The over-all zones outlined in the figure are general and not specific for grate-fired boilers. Most any modern boiler would have a primary, substoichiometric, zone for fuel gasification, after which the gas is maintained at least 1-2 seconds at about 900 °C to provide fuel-NOx reduction. After this zone, secondary air will be introduced to provide the oxygen needed for complete burnout but the temperature in the secondary zone will be controlled so as to avoid excessive formation of thermal NOx. The gas residence time in this zone will again need to be, order of magnitude, 2 seconds, or preferably a little more to provide a complete burnout.
The primary zone is β effectively β a gasification zone where the fuel bed rests on a permeable "floor" or a grate. This grate has double roles:
• It shall support the fuel bed so that it does not fall down into the air chambers
• It shall provide supply channels for the combustion air
The grate bars move back and forth pushing the fuel successively down towards the ash removal screw situated below the lowest step as shown in the photograph below.
 |
| Figure 04-00 7: The lower portion of a moving grate with the ash screw |
The picture shows the grate and part of the wall in a small "box-type" boiler with the wall lining removed during revision. This type of boilers has a simplified wall construction as compared to the tubular (panel) wall, in that the water-wall is flat on the inside. This simplified wall construction is not apt for internal over-pressure and is hence used only in hot-water boilers. Notice the anchors welded to the wall to keep the primary zone insulation in place. In the picture, they are marked with blue tape to make them clearly visible to the service personnel and to avoid personal injuries.
The two lowest steps of the moving grate are clearly visible, as is the ash screw.
The grate is made up from elements like the ones shown below.
 |
| Figure 04-00 8: The grate bars seen from the side, over-all length about 30 cm |
The top surface of the element has notches in both sides so as to provide an air passage from below. The width, the thickness, of the elements is about 3-5 cm.
 |
| Figure 04-00 9: The grate bars seen from above |
The total free area for air supply is typically between 1-10 % of the total area, different for different makes of grates.
The difference between a hot-water-boiler and a steam boiler is in the wall construction, where the steam boiler must be designed to cope with high internal pressures in the walls.
04-00-08i: District heating and CHP - liquid and gaseous fuels
The only difference between a district heating system fired with solid biofuel and one fired with a liquid or gaseous biofuel is in the boiler. You may want to refer back to text section 04-00-08f and specifically to figure 04-00 2 and its text.
While a solid-fuel boiler (see the previous section 04-00-08h and specifically figures 04-00 6, 7, 8 and-9) is designed to handle ash and the materials must be chosen to cope with any corrosive gas components and erosive ash particles that may be formed during combustion, this is very much less of a problem if the fuel is cleaned and purified through a liquefaction or a gasification process, followed by product fuel purification.
For an introduction about the production of liquid biofuels from solid biomass please refer to section 04-00-03, for liquid biofuel from liquid biomass refer to section 04-00-05 and for the production of liquid biofuel from sludge, see section 04-00-06.
Raw pyrolysis oils can be combusted using heavy fuel oil burners mounted in common oil boilers, but it must be remembered that the pyrolysis oil will contain a significant amount of the impurities originally contained in the solid feedstock. Hence, replacing heavy fuel oil with pyrolysis oil may require the installation of filters and may well lead to an increase of the wear of pumps and burners in the fuel supply system. With such fuels, there will also be good reason to modify adapt the flame monitoring system and to consider mounting constant-burning pilot burners to guarantee ignition.
Purified and clean liquid biofuels such as FAME, ethanol or methanol poses no significant process problems when replacing light fuel oil in boiler applications, but there may be reason to change the rubber quality in the fuel supply system.
For introductions on the production of gaseous biofuel from solid biomass and from sludge, please refer to sections 04-00-04 and 04-00-07 respectively.
Problems encountered when using gaseous biofuel in boiler applications are mainly concerned with the quality of the fuel gas.
If thermal gasification is used to provide a low or medium heating value gas, i.e. if an air-blown gasifier is used and the gas is cooled down for cleaning prior to the boiler, then this gas may prove a very dangerous fuel. The low heating value, in combination with a quality that is variable with the feedstock and with the actual momentary conditions in the gasifier, will be highly unstable from a combustion point of view. Such a replacement may well demand that new flame monitors are installed together with gas-fired pilot burners to guarantee a stable ignition of the gasifier product gas. The same precaution must be applied in case un-refined biogas is used as a boiler fuel.
In case a sophisticated thermal gasification process is used to produce a fuel gas of synthesis gas quality or in the case of an upgraded fuel gas from anaerobic digestion, the fuel gas may replace natural gas in almost any boiler application. However, the burners will most likely have to be replaced.
Common to all cases mentioned is that the boiler thermal capacity will most probably be reduced if a fossil fuel is replaced by liquid or gaseous. This is of course most pronounced in case the replacement fuel has an inferior quality while β in case bio-methane replaces fossil methane of FAME of transport fuel quality replaces light fuel oil β there may be no difference at all in boiler performance.
04-00-08j: District heating and CHP β planning aspects
Economy of scale and time
The initial investment in a district heating system will always be significant and if nothing else is known one may assume that the investment in the distribution network is about the same order of magnitude as that for the boiler installation. The cost for the boiler installation as a whole will be split in four parts of similar magnitude, namely the boiler itself, the boiler house, the fuel storage/handling/feeding system and the control system. To this comes the cost for the customer heat exchangers, but that cost would typically be paid by the customers themselves once they connect to the system.
To maintain a reasonably low total cost for the system it then becomes important to maintain a low variable cost in the system and to dimension it for a long lifetime. Again, the quality of the tubing β its thermal insulation and its ability to seal off oxygen β and the fuel flexibility come in as paramount parameters that have to be given due consideration during the planning and procurement phases of the project.
Generally speaking, the best situation is when there is one major customer providing a base load for the system, a base load of a significant magnitude as compared to the total capacity. Such a base load may for example be a greenhouse or maybe the official buildings of the community, a school, a hospital or a sports centre or a major shopping mall.
To judge the over-all feasibility of a district heating systems, two major parameters should be evaluated, namely the areal energy density and the potential line load.
The areal energy density is simply the total heating needs (for example measured in MWh/year) divided by the physical, geographical, size of the area where the energy measurement has been done. If, just for an example, a 1.5 km2 portion of a residential area would have an annual heat consumption of 5 GWh, then the specific areal energy density becomes 5GWh/1.5km2*year or 3.75 kWh/m2*year. For Swedish conditions, this would be considered a low number (5 or higher would be more normal) but it would not be extremely low. Suppose now that the areal considered can be shifted to accommodate also a shopping mall. Maybe the total area then becomes 2.3 km2 instead, but because of yet another group of houses and the shopping mall, the total heat consumption now becomes 17.5 GWh, raising the specific energy density from 3.75 to 7.6. So the areal energy density is not given by nature but depends on how the system is planned.
The line load is a similar measure β but instead of reflecting the areal density it will reflect the piping. The line load is simply the total energy distributed divided by the total length of the distribution network. Looking again at the above example, one may assume that in the first case (5 GWh/1.5 km2) there might be about 4-500 individual households, separated maybe 40-50 m. Assuming then 100 m (forward and return tubing) for each household, would imply a total piping of about 40-50 km, say 45. Add 5 km for the piping to connect the boiler and the line load of the system would then become 5GWh/50km or 100 kWh/m*year. This is a very low value and the consequence of such a low line load is that piping losses will become very large in this system. To be really feasible, you would prefer line loads exceeding 300 kWh/m*year but 200 might also be acceptable. For the second example above, where the area is increased to include the shopping mall and some more houses, we might assume 80 km piping just to calculate the line load and one finds 220 kWh/m*year. So again, the line load is a planning instrument β not a fixed value.
While the areal energy density and the line load are important to achieve a high efficiency in the system, they will have nothing to do with the over-all dimensioning. For this, the total heat energy demand and its annual distribution, combined in the duration graph, is the most basic instrument.
The duration graph is a diagram where the power need, W, are plotted against the number of days when this power is demanded so that one can read how many days the need exceeds any specific power. The duration graph can be drawn for the need of electricity, the need of cooling power, the need of steam or, indeed, for anything. In this case, though, it is shown for a typical case where the interest is in the need of heat for comfort heating.
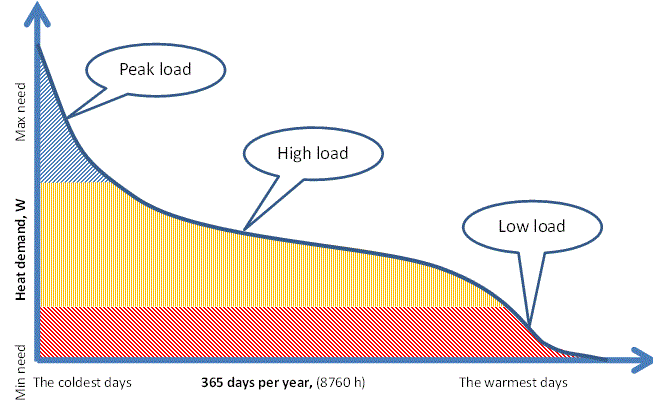 |
| Figure 04-00 10: A generic duration graph |
You will realize that the total area enclosed by the curve will represent the total, annual, energy demand for heating as expressed in Wh/year. Though the total amount of energy needed will, obviously, be a strong function of climate, the over-all shape of the graph will be similar in a great many cases.
There will be different standards in different states for how the dimensioned, maximum output from the boiler shall be treated as compared to the maximum demand.
It is important to remember that the maximum heat demand (W, comfort heat + tap water) for a system including a number of houses is always less than the sum of the maximum heat demand for the individual houses. This is because the single houses will not, in a large system with many houses, demand the peak power at the same time. Not everyone will have a hot shower at the same time.
The reduction factor to be used is the simultaneity factor (fSIM) and the way to calculate this will again be different in different climate zones. The simultaneity factor will always have a value less than unity and the maximum dimensioned power output from a boiler central in a district heating system will then be
POUT,MAX = fSIM*(Sum of peak demand in all individual houses)
To deliver this power one should not trust only one boiler unit but for safety there should always be a redundancy in the system. Also, as understood from the very brief presentation of the combustion process in section 04-00-01, there are environmental considerations to be taken into account.
For environmental, efficiency and fundamental combustion reasons, the boiler central will have to be planned to host at the very least two boiler units:
• A low-load boiler. This should be dimensioned for a maximum power output approximately equal to 25 % of the maximum output, i.e.
PLOW_LOAD β POUT,MAX*fSIM*0.25
• A high-load boiler. This should be dimensioned for a maximum power output approximately equal to 75 % of the maximum output, i.e.
PHIGH_LOAD β POUT,MAX*fSIM*0.75
At peak load, both boiler units will operate at the same time.
The maximum power output from each boiler may also be limited because of fuel availability and other reasons and there may well be a need for more boiler units in the same system β this is only the minimum. It should also be made clear that this dimensioning is valid only for solid-fuel firing and is not applicable to gas-fired or to oil fired district heating systems. With gas-firing or oil-firing, the boilers may be switched on and off very rapidly while a solid fuel fired boiler may not. Also, the formation of pollutants becomes more of a problem with solid fuels than with gas or oil, and hence the control interval for a solid fuel fired boiler becomes limited as long as environmental performance is important.
The maximum power capacity of the largest boiler is the comparative number that determines the size of the system, small, intermediate or large, as outlined in the introductory text to section 04-00-08e above.
04-00-08k: Options for large-scale applications
With a large-scale system, a more diverse product mix may become feasible because the over-all system may be able to carry higher investments.
It must be understood β however β that to maintain high over-all efficiency, either the heat demand or the fuel supply becomes the dimensioning factor.
An example:
Assume an area where β within reasonable distance β 100 000 wet tons of agricultural field residues are available on an annual basis. Assuming the reference value (see text section 04-00-01a) qNET,DAF to be 18 MJ/kg, the anticipated moisture content at delivery to be 32 % and the mean ash content to be 12 % (values chosen to resemble what can be expected in straw), the heating value at delivery will be
qNET = qNET,DAF*(1-fW)*(1-fA) β 2.443*fW = 18*(1-0.32)*(1-0.12) β 2.443*0.32 = 9.99 MJ/kg.
100*106 kg then represents 999*106 MJ or about 277.5 GWh of fuel energy.
This would approximately be a sufficient amount of fuel for a 40 MWth boiler, large enough to carry the investments for a combined heat-and-power production plant. With a modern CHP-plant one may assume that approximately half the thermal power will be available as heat, so there has to be a market for 20 MW heat to make this feasible.
Assuming that the actual market for heat is only 10 MW, one should rather go for a smaller plant β in this case 20 MWth β which might still be large enough for CHP.
So this example is to illustrate that there has always to be a market-oriented view involved when the plants are originally planned.
As a very general rule-of-thumb one may say that while a single-family heating system (< 0.1 MWth) requires high-quality pellets or briquettes for a good environmental performance, a 1 MWth boiler can be fed with wet stem-wood chips or uniform residues from a process industry. Similarly, a 5 MWth boiler can be fed with wet felling residuals or agricultural field residues.
At a scale about 10 MWth one can even feed the boiler with wet MSW (N.B. a clean fraction without heavy metals or other inorganic and hazardous contaminants) and still maintain fully acceptable environmental performance. Hence, the plants discussed in this section are larger than about 10 MWth.
Obviously, there are grey zones and the above numbers are only indicative and strongly depending on the design of the boilers but they may still serve as orders of magnitude for the combination of fuel resources and combustion technology.
04-00-08l: Large-scale: District cooling
With the rise in global temperature and the ever increasing use of home and office electronics, cooling is becoming a successively larger market and district cooling systems are a viable alternative in many places.
District cooling is exactly the same thing as district heating β only that instead of distributing hot water (at about 60-120 °C) for heating, cold water is distributed at temperatures ranging from 2-3 up to 10 °C and aimed for cooling. Just like for district heating (you may want to refer back to section 04-00-08g) the system comprises heat exchangers and pumps, a production end circuit, a distribution circuit and a customer-end circuit, all thermally insulated.
However, for the production of district cooling, heat pumps are used instead of boilers.
In a district heating system, hot water at β say 80 °C β is pumped out from the boiler central and delivered to the customers. The customers extract heat in their heat exchangers and after the water has passed all the customers it returns to the boiler central at β say β 40 °C. It is then heated again to the starting temperature 80 °C and the cycle commences.
In a district cooling system, cold water is pumped out from the cooling central at β say β 2 °C and delivered to the customers. The customers "dump" their surplus heat into the cooling water using their heat exchangers, causing the water temperature in the distribution circuit to rise. The cooling water then returns to the cooling central at β say β 12 °C. It is then cooled again to the starting temperature 2 °C and the cycle commences.
The question and the crucial point is how the cooling is achieved and figure 04-00 11 shows one of the major options, the compressor cooling machine. However, there is also another option β the absorption cooling machine.
In the most common air conditioning units, electricity is used to produce a cold medium in a compressor cooling machine. The cooling factor for such compressor units is about 2-3, meaning that if you add 1 unit of electricity, the cooling machine will have the capacity to absorb and take away 2-3 units of surplus heat. This surplus heat will, together with the electricity that was input, form a low-temperature loss that will have to be removed. To make things simple, we shall now use cooling factor 2 for the compressor machine.
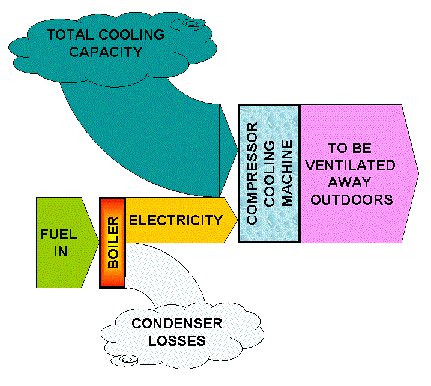 |
| Figure 04-00 11: A compressor cooling machine combined with a condensing power plant |
The combination of a condensing power plant, boiler losses omitted for simplicity, and a compressor cooling unit is outlined in this schematic.
• 1 unit of fuel energy enters the power plant and gives as a result 0.5 units of electrical energy. Here, we have assumed 50 % electrical efficiency which is unrealistically high.
• The electricity, 0.5 energy units, enters the compressor cooler, together with 1 unit of excess heat from the cooled volume. With cooling factor 2, the cooling power of the unit is exactly double to the electricity input, so 0.5 units of electricity will correspond to an absorption of 2*0.5 = 1 unit of excess heat.
• The result is 1.5 units of low-temperature heat, most often lukewarm air, to be fanned away outdoors.
In the most common application β air conditioning (AC) units β the excess heat is removed from excessively hot room air, producing cool air and requiring that the excess heat is removed from outside the building. In another common application β refrigerators β the excess heat is removed from inside the refrigerator maintaining a low temperature inside and is removed to the surrounding room, i.e. the kitchen, from the back side of the refrigerator.
Absorption cooling machines use only very minor amounts of electricity, but the main energy supply to the unit is instead in the form of heat. Absorption cooling is used in propane-fired refrigerators in caravans or for food preservation in remote areas. Since heat β regarded from a pure thermodynamic standpoint β has a much lower quality than electricity, the absorption coolers need very much more energy and the cooling factor is only about 0.5 or 0.6. Now look at the schematic below, using the cooling factor 0.5 to make things simple.
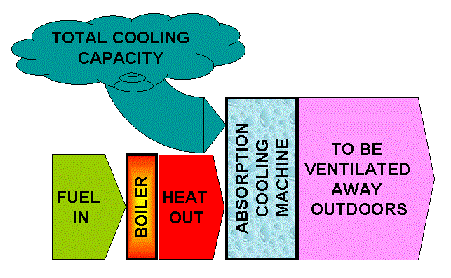 |
| Figure 04-00 12: An absorption cooling machine combined with a heating plant |
The general advantage with the absorption cooling machine is that the heat supply can be in the form of district heating water at about 60 β 90 °C while the electricity demand is low. Since hot water can be produced at very high efficiencies (well above 90 %) while electricity is only scarcely produced at efficiencies exceeding 50 %, this is an advantage.
An example:
Letβs work an example backwards to illustrate the differences and the consequences:
Assume the ultimate aim is to cool away 2 MW of excess heat from a building.
Please be observant that we will use terms like "power station" and "CHP plant" for production units that are only a few megawatt. It is obvious that this terminology is not accurate since no condensing power station nor CHP-plant would ever be built that small, but we shall still use these terms throughout the example to illustrate the different system solutions.
Using a compressor cooler with the cooling factor 2, the removal of 2 MW excess heat requires the input of 1 MW of electricity.
To produce 1 MW of electricity in a common condensing power plant with the realistic electricity efficiency 45 % one has to supply 1/0.45 β 2.25 MWth of fuel energy.
Using an absorption cooler with the cooling factor 0.5, the removal of 2 MW excess heat requires the input of 4 MW of heat to the cooling machine plus approximately 0.1 MW of electricity.
If the heat is produced in a CHP plant with a total efficiency of 90 %, then the production of 4 MW heat and 0.1 MW of electricity will require (4+0.1)/0.90 β 4.55 MWth of fuel energy.
The production mix of the CHP plant can be set to 4 MW heat plus 0.1 MW electricity β it is only the upper limit of electricity production that is fixed.
So if the aim is only to provide cooling, then the compressor cooling machine is the most advantageous from a strict fuel consumption point of view.
Stick to the same two plants, one condensing power station with the nominal capacity 2.25 MWth, electricity efficiency 45 % (i.e. 1 MW electricity output), and one CHP plant with the thermal capacity 4.55 MW, total efficiency 90 % whereof maximum 38 % electricity.
Now consider the situation that there is instead a need of only 1 MW cooling power.
The first system β the condensing power plant combined with compressor cooling β now has two main options:
1) Run at half load, i.e. at about 1.1 MWth and supply only the compressor cooler.
2) Run at full load, i.e. at 2.25 MWth, supply the compressor cooler 0.5 MW electricity and deliver/sell the remaining 0.5 MW electricity to the main grid.
The second system β the CHP-plant with its absorption cooler β has several options but suffice it to outline only two:
1) Run at half load, i.e. at about 2.3 MWth and supply only the absorption cooler.
2) Run at full load, i.e. at about 4.55 MWth, and maximize the electricity production (i.e. 38 % of 4.55 MW = 1.73 MWel). Deliver sufficient heat (2 MW) and sufficient electricity (assume 0.1 MW, the electricity demand for absorption heat pumps is not directly linear with cooling demand) to the cooling machine. Cool away (i.e. dump) the surplus heat (0.36 MW) and deliver/sell the remaining electricity (1.73 MW subtracted by 0.1 MW for the cooling machine = 1.6 MW) to the grid.
You will notice how the system including a CHP plant with an absorption cooling machine in this second case, as the cooling demand decreases, successively switches to become not only a provider of cooling but also a significant producer of electricity, delivering to the grid more than three times the power that the condensing power station can manage.
The price paid for this advantage is that the CHP plant must originally be dimensioned bigger (4.6 MWth) than the condensing power plant (2.25 MWth). The product mix flexibility inherent with the second system becomes even more pronounced in such cases when there is β as is often the case depending on how the planning has been made β there are simultaneous demands or heating and cooling with different customers.
In this example we have assumed that the production plants (the "power station" and the "CHP plant") were both dimensioned so as to, at full load, only be able to supply the cooling machines and obviously this is never the case in real life but the example is only here to illustrate the over-all differences between the different system solutions.
You will also notice how the total loss from the CHP plant (boiler loss 10 % of 4.6 = 0.46 plus 0.36 MW of surplus heat that is cooled away in this case) is only slightly more than the total thermal loss from the condensing power station (0.6 MW) in spite of the fact that the size of the CHP plant is more than double (4.6 MWth as compared to 2.25 MWth).
It must now be clearly stated that these technologies β CHP, condensing power production or any other type of electricity production, compressor cooling machines and absorption cooling machines β are by no means exclusive. They all have a role to play in a sustainable, future energy supply system. But the two (CHP and condensing) are based on completely different philosophies with quite different consequences when cooling production and cooling delivery is taken into account:
• With condensing power production, the idea is to produce electricity and nothing else and any cooling produced by compressor cooling machines provides an electricity demand reducing the amount of electricity available for other purposes.
• With CHP, the idea is to use the need for heat as a basis for electricity production and any cooling produced by absorption cooling machines provides a heat demand making it possible to increase the electricity production.
Hence the net electricity production capacity of condensing power plants is reduced as cooling needs increase (i.e. during summer and hot weather) while CHP-plants combined with absorption cooling machines and district cooling networks have the opposite characteristic!
Since cooling machines are expensive, both solutions are feasible only in large scale but it should be remembered that district cooling production in modern CHP-plants provides a very good basis for electricity production and also offers the flexibility to sell heat, to sell cooling and to sell electricity on a seasonal market.
04-00-08m: Large-scale: Co-firing
As was briefly mentioned in section 03-00-02d, solid biomass can be milled together with coal and co-fired in pulverized coal power stations. In many cases this may be the simplest and cheapest method to introduce biofuel into the national energy system. Experience from several types of plants and installations have for example been reported and documented by the International Energy Agency (IEA), clearly demonstrating the feasibility of this technology.
The types of mills most commonly installed in this kind of applications are ball mills or rod mills. To achieve the size required for pulverized fuel firing, i.e. < 0.8 mm approximately, in this type of mills, the material must be mechanically brittle. Biomass is usually not brittle β unless it is very dry. Hence, to make the material suitable for grinding, it must be pre-treated.
Due to the difference in ash- and combustion properties between coal and biomass, and also because of the lower heating value with biomass and hence the large amounts to be handled, it is usually not advisable to go beyond 10-15 % of the total thermal input. However, most experience indicates that biomass input at these levels causes no major operational or environmental problems with the power stations.
04-00-08n: Large scale: Planning aspects
The upper scale of biomass-based energy plants will β in most cases β be set by the availability of the biomass. As was pointed out in chapter 02-00, sections 02a and 02b, the natural growth rates of biomass is limited to, order of magnitude, less than 15 tons of dry substance per hectare and year. Considering that the typical dry substance heating values are again limited to, order of magnitude, 20 MJ/kg, one then finds that an approximate areal energy production density would be 15*103*20 = 300 000 MJ/hectare and year corresponding to a bit more than 83 MWh/hectare and year or 9.5 kW/hectare. This is then an upper limit and more realistic would be 10 % of this.
Using an average growth rate of 1 kW/hectare for simplicity, one will now realize that a plant with the average thermal load 10 MW (i.e. 10 000 kW) needs a fuel supply collected across 10 000 hectares corresponding β if a perfect circle is assumed β to a radius < 6 km. On the other hand, a 1 GWth (about 450 MWel if a common steam cycle is assumed) power station would need to be supplied with the biomass inside about 56 km radius.
As has been pointed out at several places throughout this chapter, the differences in fundamental properties between different categories of biomasses will affect their usability with different processes.
Hence, the use of biomass in large-scale combustion applications will be limited by the transport distances needed to provide the plant with the kind of biomass it was designed for. For the 1 GWth power station mentioned above, this means that only the biomass of the correct category can actually be used which will expand the radius to maybe 100 km, depending on the structure of the area.
There is also another aspect to the size of biomass-based energy plants. Because of the low energy density and the corresponding transport cost (text section 03-00-02b), solid biofuel may become comparatively expensive and thus demands that the processing provides the highest possible total efficiency. Hence, biomass-based energy plants should preferably be CHP-plants. But then there is the question of the heat market. Going back to the case of a 1 GWth power station and assuming it to be a super-modern combined-cycle BIGCC (04-00-08f) one may assume maximum electricity efficiency about 62 % yielding a heat production capacity exceeding 300 MW. So it also becomes a question of finding a sufficiently large heat market.
| The over-all conclusion and recommendation for the initial stages of planning thus becomes first to identify and categorize the resources at hand in the region, then to identify the market for heat sales and to use these data to decide the scale of the plant. |
04-00-09: Processes demanding fuel conversion
Some types of combustion equipment are in themselves very sophisticated or specialized. In such cases a conversion of the solid biomass into a gaseous or liquid fuel may be necessary prior to the final use of the energy. Processes or equipment subject to these constraints are:
• Internal combustion engines since they require very high combustion intensity and cannot in a simple way be built to remove ash from inside the combustion chamber (i.e. the cylinder). For stationary applications such as small-scale CHP (04-00-08f), the fuel quality, whether gaseous or liquid, may not be of paramount importance since the weight of the engine is not crucial, but for transport applications the quality is a determining factor.
• Gas turbines where corrosive or erosive gas components may prove disastrous to the smooth surfaces required for high efficiency as well as to the fine-tuned balance required for turbines operating at 3000 rpm. Gas turbines were already touched upon in text section 04-00-08f above.
• Commercial fuel cells are today limited basically only to extract the energy from the hydrogen in the fuel and are very sensitive to catalyst poisoning. Some fuel cells can be fed with methane or other well-defined, lighter hydrocarbons, and are capable of extracting the energy not only from hydrogen but from the carbon too, but so far these cells are not fully commercial.
• Industrial processes where the product is in direct contact with the flue gases and at the same time is sensitive to contamination. Just to choose a very simple example: You would not want the hot combustion gases from solid biofuel, containing a bit of soot and solid ash particles, to be brought into contact with the white paper at the drying end of your paper mill. In this case, and in similar cases, there are two options: The process may be re-designed for indirect heating or the fuel may be converted and purified into a clean fuel.
• Industrial processes demanding temperatures above 1500 °C. In these cases, a fuel conversion in itself is not sufficient but a fuel upgrading is also necessary. Unless the ballast in form of water vapour and inert gases are removed, gasification of biomass does not in itself improve the fuel quality or raise the attainable flame temperature. The same thing holds true for any conversion. Dry and solid biomass β like woody biomass β may well be used to achieve temperatures exceeding 1500 °C but for practical purposes β simplified process control systems and more accurate control β it is often preferred to use liquid or gaseous fuel for these applications.
04-00-09a: Conversion: Transport fuel
In recent years the production of transport fuels from biomass has been a major research area. For transport fuel there is one major demand and that is for the heating value (04-00-01a) and/or the energy density (03-00-02b) β depending on the vehicle:
• Trains, with the very low friction and high load capacity, are much less sensitive to the weight and volume of the engine itself and of the fuel carried than aeroplanes.
• Private cars, where you want to have a large passenger and luggage compartment, require that the volume necessary for the fuel carried shall not be too large. Nor may the engine be too heavy.
• Lorries and trucks, where the demand may rather be for a high weight carrying capacity, will instead demand that the weight of the fuel is not too big.
Hence, the demand on the fuel becomes different though there is a common denominator, namely that the fuel must be of sufficiently high quality with respect to energy content.
The second demand put onto the fuel is that it shall be suitable for the engine used.
The two major types of engines β the Diesel engine and the Otto engine β mainly differ in the way the fuel is ignited:
• In the Diesel engine, the fuel is ignited by the heat developed as the air inside the engine cylinder is compressed. This type of ignition is β within limits, of course β robust with respect to the fuel composition and such engines may thus be run on a number of fuels. For optimal performance, the engine must be adjusted to the fuel but generally speaking, Diesel engines are more fuel tolerant than Otto engines.
• In the modern Otto engine as used in most gasoline-supplied cars, the fuel is ignited by a small electric discharge (a spark) from a spark plug. The ignition energy supplied from the spark plug is immensely less than that available by the compression in a Diesel engine and hence the fuel must be such as to actually ignite in response to the spark. This puts strong demands on the fuel quality and Otto engines are much more limited with respect to the fuel properties than Diesel engines.
Common to both types of engines is that with an engine running at β say β 6000 rpm, equivalent to 10 ms per revolution, the fuel must burn out completely within half a revolution i.e. within 5 milliseconds. Hence, in both cases, the fuel must be fast burning. This requirement could be eased by using engines running at low rpmβs but the price paid in this case is a significant increase of engine weight.
For the Diesel engine, it is important that the fuel actually ignites when the temperature and the pressure in the cylinder increases. Depending on the fuel quality, this ignition may take shorter or longer time and this time is classified using the cetane index or the cetane number. The demands on Diesel fuels to be marketed within the European federation are set in the standard EN 590. The cetane number and its connection to the raw materials for biodiesel production is slightly touched upon in the text section about fame.
For the Otto engine β on the contrary β it is important that the fuel does not ignite spontaneously during the compression phase. The resistance to compression ignition is quantified using the octane number and the higher the octane number, the more compression the fuel can withstand without igniting. Hence high octane numbers are strived for in fuels aimed for Otto engines and for aeroplane engines. The European federal standard for gasoline is EN 228.
Both engine types may β provided the fuel injection is adjusted accordingly β be run on gaseous fuels. However, since the engine must be adjusted to fit the fuel quality, the fuel must then be guaranteed to keep that quality over time.
Hence, the production of transport fuel from biomass demands not only a strict quality control but also process stability so that the same fuel quality can be maintained in spite of any variations in feedstock. This limits the flexibility with respect to feedstock and increases the economic vulnerability of the whole process.
It is also important β already during the planning process β to take the demands put in directive 2009/30/EC into account. This directive specifies some of the demands put on greenhouse gas emissions and process documentation put on the production of transport fuels.
04-00-09b: Conversion: Fuel cells
The attractive side of fuel cells is their capacity to directly β without any internal mechanical or moving parts β transform chemically bound energy in a fuel into electricity. Indeed, this is what also happens in a common battery but the word "fuel cell" is commonly used only for such processes where the electrolyte is continuously fed to the cell from the outside. In a common battery, everything β electrolyte and electrodes β is contained in a sealed unit and the end products do likewise remain inside the container. In a fuel cell the electrolyte is pumped or β in case of a gas β pressed into the cell and the end products are extracted continuously.
Fuel cells can β very crude β be separated into low-temperature fuel cells and high-temperature. The high-temperature fuel cells operate above about 500 %deg;C and include molten carbonate cells, solid oxide cells, ceramic cells and others like the direct carbon fuel cells. With high-temperature cells it becomes important not only to market the electricity produced but also the heat and high-temperature fuel cells do therefore compete with other CHP technologies as they are outlined in text section 04-00-08f and the following.
The current development of fuel cells aims at high power units suitable for commercial power production at a high efficiency but so far (2012) the over-all economy of such systems is prohibitive as compared to other technologies.
The major application for fuel cell technology is instead in remote installations where the absence of any mechanical and moving parts, and hence the low maintenance cost, is a major advantage. However: in combination with a complex biomass conversion and fuel purification process, this advantage no longer exists.
Hence, the use of fuel cells in combination with biomass puts high demands not only on the fuel conversion but also on the subsequent fuel cleaning and purification. Therefore, the advantage of the fuel cell β itβs mechanical simplicity β is outflanked by the complexity of the biomass conversion and fuel purification and the system hence must compete with the other technology options outlined in 04-00-08 only on its economic merits.
04-00-10: Flue gases and emissions
Though biomass in general holds low concentrations of sulphur, the concentrations of nitrogen as well as of chlorine may sometimes be high.
Also, the ashes may contain high concentrations of high-volatile metals, especially the alkaline metals like sodium and potassium.
The flue gases from biomass and biofuel combustion may thus have high concentrations of small (sub-micron) particles and biofuel-fired plants may require advanced particle clean-up of the flue gases to meet high environmental standards.
The relatively high concentrations of nitrogen may also be cause for high emission levels of nitrogen oxides, again calling for advanced combustion control and in some cases for additional de-NOx installations.
The fact that biofuel are carbon-dioxide neutral and "natural" does hence not guarantee that the use of biofuel in inadequate equipment becomes environmentally friendly.
The demands on flue-gas cleaning may vary between installations since the ultimate demands as set in the federal directive
2008/50/EC puts the emphasis on the outdoor air quality and not
specifically on the flue gas quality. Hence, the size and the localisation of the individual plant as well as
other sources of pollutants in the vicinity will all have an impact on the demands put on the specific installation.
Generally, though, the federal directive
2010/75/EU on Industrial Emissions sets specific limits for
combustion units and several types of fuels.
Since 2012, the Industrial Emissions Directive
(IED) 2010/75 is effective. This directive sharpens the demands
on combustion plants in general and a more detailed description of the consequences will be included in a
special text section here.