The ash composition
Ash is the general term used to describe the inorganic matter in a fuel. In biomass fuels, the ash content may originate from the biomass itself, e.g. materials that the plant absorbed from the water or the soil during its growth, or from the supply chain, e.g. soil collected along with biomass. In any case, after the collection of a sample the ash content is typically measured by combusting the biomass at a laboratory furnace under controlled conditions, taking into account the relevant standard EN 14775.
It is important to notice that the ashing temperature for biomass fuels is 550 °C, lower than the typical ashing temperature for coals, which is 780 °C, The reason is that biomass ash contains several volatile elements which may not be present in the laboratory produced ash if it is combusted in high enough temperatures. Thus, high temperatures for ash mean a non-representative ash sample, both in terms of quantity and quality. It is also fairly obvious that ash collected from real-life application will differ in some extent in composition from the fuel ash prepared in the laboratory.
Generally, the ash content of herbaceous biomass is higher than that of woody biomass. While ash weight content (in dry basis) values of less than 1% are expected for wood, different herbaceous biomass types have reported values ranging from less than 2% up to 8 â 10 % or even up to 25% for rice husks. In waste fractions, the ash content may often be as high as 30-50 % and is only scarcely less than 10 %. However, in waste streams, the ash may well be constituted by glass, ceramics and other substances that have had a household or industrial use but have been erroneously sorted into the combustible fraction.
Apart from the supply chain impact, the ash content is heavily dependent on plant type and growing conditions. Water uptake for example is directly related to uptake of Si and other inorganic elements in the plant. Thus, C3 plants which have on average higher water uptake compared to C4 plants also tend to have higher ash contents. (you may want to refer to section 01-00-02a).
However, some C3 plants such as cardoon have been widely reported to have an ash content of 8% wt db or more. The use of fertilizers also affects the ash content in herbaceous biomass, particularly with regard to elements such as potassium, chlorine and phosphorus.
Soil conditions and âcomposition also plays an important role in virgin biomass. Some researchers have indicated that switchgrass growing on sandy soils resulted in lower overall ash and potassium content compared to switchgrass grown on clay soils. Therefore, the level of soluble inorganic elements in a soil is important.
Finally, the ash content and composition of a plant tends to vary depending on the part of the plant. Leaves for examples typically contain higher amounts of ash than stems.
Following the preparation of a laboratory ash sample, European Standards exist for the determination of two main groupings of elements: minor elements and major elements.
Minor elements should be measured according to EN 15297. They include the following elements: As, Cd, Co, Cr, Cu, Hg, Mn, Mo, Ni, Pb, Sb, V and Zn. The term âminorâ stems from the very small concentrations in which they are typically found in the fuel. Their measurements relates mostly to environmental concerns, e.g. whether the ash contains any toxic elements, such as mercury, chromium or cadmium, that may return to the environment upon its disposal. Other elements, such as Zn, may have a role in the formation of aerosols or a minor impact on fouling issues. Minor elements are typically expressed as (mg / kg) of fuel on a dry basis.
Major elements should be measured according to EN 15290. They include the elements which are most abundant in the fuel ash: Al, Ca, Fe, Mg, P, K, Si, Na and Ti. No significant environmental issue is associated with these elements, however they have a major impact on the ash melting behaviour, slagging/fouling and corrosion. High concentrations of some elements may indicate fuel contamination with soil or sand.
The most important of the above elements are important plant nutrients: Si, Ca, K (the most important macronutrient after nitrogen), Na and P. There are several ways to express major elements: (mg/kg) of fuel on a dry basis or as weight percentages in the fuel ash. A tradition left from the use of empirical indices originally developed for coal is to express the concentration of major elements as their corresponding oxides. The conversion of the weight concentration of a major element to its oxide is easy if one uses the ration of the oxide molecular weight to the element atomic weight, as described in EN 15290.
Weight concentrations of major elements vary greatly between biomass species. Typically, herbaceous biomass has higher concentrations of silica and lower concentrations of calcium compared to woody biomass. In addition, the alkali concentrations (K and Na) of herbaceous biomass is quite high. The combination of high silica and alkali content is especially problematic, as will be described in section 04-02-02a and 04-02-02b. Literature sources provide a wide range of values, however the impact of growth conditions and supply chains result in quite different reported for the same biomass type.
Another type of measurement that can be performed in the fuel ash is the determination of the ash melting temperature. This will also be discussed in more detail in section 04-00-01.
Other methods to describe the ash
Apart from the measurements described in the standards, a common laboratory characterization of fuel ash is the determination of mineral composition through X-Ray Diffraction (XRD) Spectroscopy.
This method may reveal the presence and relative concentration of different mineral phases. For example, it can be determined whether potassium is chemically bound with chlorine in the form of sylvine or with sulphur in the form of arcanite. There are no standardized guidelines on how to evaluate the results of an XRD analysis for biomass, but if such info is available it can be used by experienced technicians and engineers for the design of a better system as certain minerals are associated with erosion or slagging issues.
The European Standards for solid biofuels also include EN 15105 âSolid biofuels - Determination of the water soluble chloride, sodium and potassium contentâ. Chlorine, potassium and sodium are commonly found in the form of water soluble salts which, during combustion, are readily released in the gas phase.
An analysis according to this standard may therefore provide an indication of the aggressive content of these elements, in relation to potential slagging and fouling problems. In addition, such chemical are more ease to remove by leaching the fuel. Although in some biofuels the water soluble content of these elements is practically the same as the total content, this is not always the case. For the total content, standards EN 15290 and EN 15289 apply.
Slagging and fouling
In combustion systems, the heat released from the fuel oxidation is typically transferred to a working medium, such as steam or hot water, which is used for power, heat or cooling demands. The hot flue gas is not in direct contact with the working medium; the energy transfer is achieved through heat exchanging equipment, such as tubes. Optimally, both sides of the tube should be clean in order to achieve the maximum rate of heat transfer under the conditions. In practice though, deposits do get formed, especially on the flue gas side. This problem is most common in solid fuel combustion systems, due to their high ash content compared to liquid or gaseous fuels.
Deposits add a thermal resistance between the flue gas and the working medium and as a result reduce the heat transfer between them. Thus, the flue gas exits the combustion system at higher than the design temperatures and energy losses to the environment are increased. Deposits also tend to accumulate over time and, if the flue gas path is narrow enough, they may even block it completely. In some cases, after the accumulation of significant deposits, chunks may fall off and damage the interior of the boiler. All in all, deposits tend to decrease the efficiency of a combustion system and increase the operating and maintenance cost.
The deposit problem is typically classified in two broad terms: slagging and fouling:
Slagging refers to deposits formed on sections of the boiler exposed mainly to radiant heat, such as the furnace walls. Slagging deposits are formed from molten or half molten ash particles that stick to the hot furnace walls. They are not formed immediately upon firing up the boiler but accumulate slowly after an initial layer has been formed over the walls.
Fouling is used to characterize the deposits formed on the convective pass, such as the heat exchanger tubes. In this case, deposits are formed by inorganic vapours that condense on the relatively cooler surfaces of the heat exchanger tubes. Although the mechanisms of formation for slagging and fouling are not the same, both are closely linked with the tendency of the fuel ash components to melt or vaporize at low temperatures.
The ash melting behaviour is greatly affected by the ash composition. Typically, elements such as calcium and magnesium increase the ash melting temperature, while silica, potassium and sodium decrease it.
It is therefore evident that herbaceous biomass ash is expected to have low ash melting temperatures while woody biomass generally will exhibit high ash melting temperatures.
In practice, it is not only the alkali content of herbaceous biomass that affects its fouling behaviour but also the chlorine content. Alkali chlorides facilitate the transport of alkalis in the gas phase, are very volatile and are released in the gas phase in the combustion zone; in the convective zone, they condense on the cooler surfaces as can be seen in the figure.
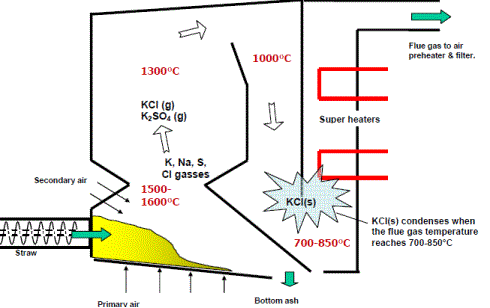 |
| Figure: Fouling by KCl in a fixed bed straw boiler (Source: DONG Energy) |
The condensed ash particles are molten and form sticky deposits that tend to enhance deposition of even coarse fly ash particles that would normally bounce off the surfaces. The presence of alkali chlorides also decreases the overall ash melting temperature to values between 700 â 800 °C or lower.
Estimating ash deposit behaviour I
There are two main ways to estimate the tendency of a biomass ash to form deposits. The first method is the direct determination of the ash melting (or fusion) temperature. A biomass ash sample is pressed into a test piece with specified dimensions in inserted in a controlled furnace and its decomposition as temperature increased is monitored constantly. There is not yet a European Standard, only a Technical Specification, CEN/TS 15370-1 â Solid biofuels - Method for the determination of ash melting behaviour - Part 1: Characteristic temperatures methodâ. Another commonly used standard is the ASTM D 1857.
Although the exact temperatures to be recorded and definitions thereof differ from the standard, the following may be used for the purposes of this handbook:
• The Shrinkage Starting Temperature (SST) is the temperature at which the test piece first starts to shrink, possibly due to the release of volatile inorganic materials or due to sintering. This temperature value is not a part of the common ASTM standard so it is not usually recorded in older data.
• The Initial Deformation Temperature (IT) is the temperature where tips of test piece show the first signs of rounding due to melting.
• The Softening Temperature (ST) is the temperature where the height of the test piece is equal to its width. This temperature is not included in the European Technical Specification, although it is commonly reported.
• The Hemisphere Temperature (HT) is the temperature where the test piece has melted sufficiently to form a hemisphere.
• The Flow Temperature (FT) is the temperature where the test piece has effectively melted and is spread over the supporting tile.
A well-known and widely used index based on the temperatures above is the slagging index, which is calculated from the following equation:
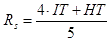
The higher the slagging index, the lower the propensity for formation of strong slagging deposits. Generally, fuels with a slagging index less than 1150 °C have a severe slagging potential, while values greater than 1340 °C indicate weak slagging potential. Values in between cover a wide range of severe to moderate slagging potentials.
For biomass fuels, the IT is also considered a valid indication of the tendency of the ash to cause problems during combustion.
The measurement of ash melting temperature provides a direct correlation between laboratory data and the tendency of an ash to melt. However, it offers no information on the ash composition and the transformations that the ash may actually experience in the combustion chamber.
Estimating ash deposit behaviour II
The second way of evaluating the propensity of a biomass ash to form deposits is the use of appropriate indices, based on its major element ash composition (see The ash composition above).
Several ash indices have been developed, initially for coals, and are known to be quite accurate in their predictions. However, due to the different composition and morphology for the ash, their value for biomass fuels is contested.
New indices are being developed for biomass fuels, although their general applicability is not yet completely accepted. Some indices that have been developed include the molar ratio Si/(Ca+Mg) or its modified version (Si+P+K)/(Ca+Mg) for fuels containing high amounts of phosphorus as is the case with several types of herbaceous biomass. Since calcium and magnesium tend to increase the ash melting temperature, the higher this index, the lower the ash melting temperature and the fouling propensity.
There are a great number of different indices and key numbers such as the Miles index, the alkalinity indices, salt ratios, glass formation index and a number of others â none of which gives the full picture or a definite answer to slagging and fouling behaviour. It must also be understood that depositions occur in such places inside the combustion or gasification reactor where conditions â oxygen content, temperature and gas velocities â happen to be favourable. Hence, any evaluation of average or mean conditions may only be seen as an indication and cannot be used to predict in any detail where, when or to what extent depositions will occur.
The application of the different indices, as well as the interpretation of their meaning, should therefore be left to experienced technicians or researchers. However, the indices, blunt as they are, once again pinpoint the importance of several elements such as K, Si and P in the general description of ash melting behaviour.
The reader will notice that chlorine is absent in those indices despite the fact that, as was mentioned earlier, it is extremely important in the fate of potassium in the furnace. The effect of chlorine will be discussed in the following section on corrosion.
Ash utilization
Apart from some minor loss of inorganics as aerosols or dust particles, most of the biomass ash in combustion applications is collected either as fly ash, which is collected at the ash cleaning equipment along the flue gas path, or bottom ash.
Although the ash quantities that are produced by herbaceous biomass combustion are usually not comparable with the amounts of ash that several types of coal produce, nonetheless finding utilization pathways for such a combustion residue is an important aspect of any combustion system, if only to maintain its environmentally friendly outlook. Despite this, the issue of ash utilization is in several cases omitted from the design phase and postponed until the installation is operating.
Two main reasons contribute to this:
first, the landfilling option can in most cases be chosen, even though it may not be the cheapest option;
second, the composition and properties of combustion ash differs depending on the actual fuel quality combusted as well as the installation type.
For coal ash, the issue of ash utilization in most cases has a relatively easy answer: itâs used as an additive for the production of cement or concrete. However, many biomass ashes will contain much higher concentrations of alkali than the standards which regulate the use of coal ashes allow. Thus, the utilization of biomass ashes in concrete/cement production is only possible if the ash comes from a co-firing installation (see 04-02-03) and even in this case, the biomass thermal share must be quite low.
Many biomass ashes are rich in potassium, which is an important plant nutrient. Potassium is easily leached from the ashes and becomes available for plants. Other plant nutrients, such as calcium, may also be present. Thus the re-cycling of biomass ashes as a fertilizer is a major option. There are some issues that need to be considered in this application though:
• Fly ashes from biomass combustion may be rich in potassium, but most of the nitrogen has been released in the gas phase. In addition, phosphorus may be present but in an insoluble form, which make take several decades before it becomes accessible to plants. For forestry this may be no issue due to the longer growth cycle but for agricultural practices, other nitrogen and phosphorus containing fertilizer may still have to be applied.
• Fly ashes also tend to be rich in all the heavy metal contaminants, such as cadmium, that a biomass fuel may contain. The biomass fly ash must meet certain requirements regarding heavy metals concentration before its use as a fertilizer may be considered. This is particularly relevant in cases when biomass is co-combusted with other, heavily contaminated fuels.
• Finally, due to the volatile nature of alkalis, bottom ashes may exhibit rather low potassium concentrations and thus their return as nutrients in the field might make little sense.
The question of how a system planner should approach the issue of herbaceous biomass ash utilization is addressed in more detail in section 04-02-02a.
Estimating corrosion behaiour
Corrosion occurs when the protective oxide layer that is formed on tube walls is attacked primarily by chlorine or sulphur containing compounds. The sulfidation and chloridation of the tube surfaces results in the formation of an outer layer that does not have the protective properties of the oxidised one. Its defective structure means that it can be scaled off easily due to erosion and thus become subject to further corrosion.
Thus, corrosion refers to the deterioration of intrinsic properties of the wall or tube material. Contrary to slagging/fouling, which can be controlled to some extent by cleaning procedures, such as soot-blowing even while the boiler is operating, corrosion is permanent and severely affects the lifetime of the equipment.
Chlorine corrosion is particularly relevant for the combustion of herbaceous biomass and waste fractions or refuse-derived fuels. It can occur through either one of three main mechanisms:
• Gas phase corrosion, which is the direct attack of gaseous HCl or Cl2 to heat-exchanger surfaces;
• The formation of alkali sulphate and/or alkali chloride melts, which dissolve the protective oxide layer of the heat-exchanger surface
• The active oxidation mechanism which refers to the sulfation of alkali metal or heavy metal chlorides in the tube near the deposition layer. From this mechanism, Cl is released, which subsequently attacks the tube surface.
Active oxidation is the most critical mechanism regarding high-temperature corrosion. As can be seen, its extent relates mostly to the formation of alkali chlorides deposits on tube surfaces. As was mentioned before, most of the alkalis in herbaceous biomass high in chlorine are typically released in the gas phase as alkali chlorides, it is clear that fouling and corrosion are closely linked in herbaceous biomass and the extent of fouling is a good indicator of the corrosive properties of a fuel.
A number of measures can be taken to reduce the corrosion potential. These include:
1) Reducing the chlorine content of the fuel. For herbaceous fuels this can be achieved via leaching during storage, as discussed in section 03-02-xxxx. With waste fuels, the content of chlorine is mainly determined by how well the fractioning and separation of PVC works in the waste collection system. Both ways, the overall chlorine that enters the combustion chamber is reduced.
2) Proper design of the combustion chamber. If the gas temperature where alkali chlorides are brought
into contact with the tube surfaces is low enough, the deposits are not molten but solid and their corrosion
potential is reduced. Such a temperature would be about 650 °C. This is a common design in waste incinerators.
The price paid is a reduction of the electricity efficiency of the plant as discussed in text section
04-00-08f. Also, special coatings or steel alloys for the construction of the tube surfaces may be used, although this increases the capital costs of the boiler.
3) Use of additives or complementary fuels that influence the combustion conditions towards the formation of compounds with lesser corrosion potential. The general idea would be that the alkali content of the biomass ash would react with certain other elements and become bound in structures without chlorine. Thus chlorine would end up in the gas phase as HCl, which is less critical for corrosion, while the alkali compounds would either have higher melting temperatures and/or lower corrosion potential. Such additives fall in one of three categories:
• AlSi additives, such as kaolin and dolomite
• Phosphorus containing additives, such as dicalcium phosphate
• Sulphur containing additives, such as ammonium sulphate.
The molar ratio (Al+P+2S)/(K+Na) for the fuel plus additives indicates whether there is sufficient additives to capture the alkali. In practice, ratios greater than 1 are required.
Unfortunately, most commercial additives are quite expensive and have a heating value of zero or negligible. A fuel price increase of up to 20 % can be expected in many cases. Hence, it is preferable to co-fire herbaceous biomass along with fuels that naturally contain one of the three additives or high concentrations of the critical elements.
The evaluation of the corrosion potential for herbaceous biomass can be performed again using certain empirical indices. For example, the sulphur to chlorine molar ratio is calculated as 2S/Cl. The molar concentrations of each element can be found by dividing its weight composition in the fuel by its molecular weight.
Fuels for which this ratio is high tend to form a protective sulphate layer on the tubes. If the ratio exceeds 4, only minor corrosion is expected - with values over 8 the chlorine presence in the deposits is minimal. This explains the tendency to co-fire herbaceous biomass along with fuels containing high concentrations of sulphur, such as certain coal types or peat.