Descriptions of the biomass fuel composition
Biomass for energy can generally speaking originate from trees and bushes (woody biomass or ligno-cellulosic biomass), from herbs or flowers (herbaceous biomass), from fruits or nuts (fruit biomass) or from industrial or societal activities including households (refuse or waste, sludge is also included in this group).
Global terms
Different types of biomass will have radically different properties but they will all be described using a common terminology, a terminology that is introduced in this text section and further discussed in chapter 04-00.
• The "as received" composition (ar) includes the moisture content of the biomass, typically at the point of harvesting or delivery. It is the most valid composition to be used in terms of performing combustion calculations, estimating efficiencies, etc. On the other hand, the exact as received composition is obviously affected by the moisture content and all the factors which affect it. Also, the moisture content is the most easily controlled quality parameter of the fuel, being subject to change through drying processes. As a result, the moisture of a sample may vary between the sampling point, the delivery point and the final analysis in the lab. Therefore, although extremely useful for actual applications, the as received composition is not typically a valid indication for comparisons between biomass types.
• The "dry basis" composition (db) refers to the composition of the biomass excluding all water content. Obviously, this state can only be applicable for laboratory samples – all other types of drying in "real-life" applications always leave some of the moisture, however low, in the fuel. The dry basis is a good starting point for comparing the properties of different fuel types and is the typical format in which most laboratories report their results. Several types However, it does not take into account variations in the inorganic part of the biomass which may be due to the impact of the supply chain.
• The "dry, ash free" basis (commonly abbreviated as "daf") refers to the composition of biomass excluding all water and ash content. The dry, ash free basis is an even more ideal case than the dry basis, since actual separation of the ash from the organic part of biomass is impossible – in the laboratory and combustion applications, it is the organic part that is separated from the ash. However, this basis allows for the exclusion of all influences of the supply chain and for the direct comparison of the properties of different types of biomass fuels. The daf basis is used for example in Chapter 03-00.
This terminology is common to all biomass types, and indeed for any solid fuel including coal, and the typical breakdown of the main components of biomass into over-all categories is: moisture content, organic (or combustible) content and inorganic (or ash) content.
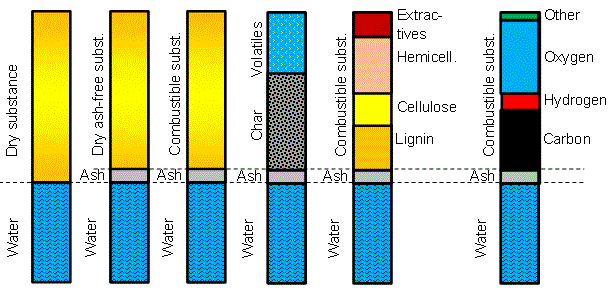 |
| Figure: Terminology in biomass analysis, also applicable for other solid fuels |
Proximate analysis – Char and Volatiles
A more typical way of categorizing the organic composition of biomass is what is commonly called the proximate analysis: the breakdown of the fuel in volatiles, char or fixed carbon and ash. As discussed in Chapter 04-00, the proximate analysis is typically given on a dry basis and should be measured in a laboratory employing the relevant European standards (EN 15148 for volatiles, EN 14775 for the ash content).
The content of fixed carbon, "char", is calculated by subtracting from 100 the weight composition of the other two compounds. Typically, controlled laboratory furnaces or specialized equipment such as thermogravimetric analyzers are used for the analysis.
Again, most biomass will have a higher volatile content than coals. Herbaceous biomass also tends to have slightly higher volatile content compared to woody biomass or certain agro-industrial residues; a general trend would be that the lower the lignin content, the higher the volatiles a biomass has. With waste biomass, depending on the fraction, the volatile content can be as high as 90 % of daf, with herbaceous it is usually in the range 70-85 and with woody biomass it is usually about 60-80 % of daf. A more detailed discussion of the effect of volatile composition on combustion applications can be found in section 04-00-01.
Cellulose, Hemicellulose, Lignin and Extractives
Being a product of the photosynthetic process, as discussed in Chapter 01-00, the dry matter content of herbaceous biomass resources are also typically composed of carbohydrate polymers (cellulose and hemicellulose), phenolic polymers (lignin) and, in lesser quantities, other substances, such as resins, fats and fatty acids, commonly known as extractives.
The measurement of the cellulose, hemicelluloses and lignin content of biomass is not a standard analysis, especially for combustion applications. This is evident by the fact that there is not a European standard for it.
The distribution of cellulose, hemicellulose, lignin and extractives is most relevant in the case of liquid biofuels production and in cases when biochemical conversion processes such as anaerobic digestion and/or fermentation are applied. Lignin is the most difficult substance to digest and may, if present in too large amounts, cause problems or at least a drop in total conversion performance in anaerobic digesters while cellulose is not easily fermentable and may cause problems in the case of ethanol production plants. The standards typically employed either come from the paper & pulp industry (the TAPPI standards) or are ASTM standards (e.g. ASTM E 1758-01).
One key difference of herbaceous biomass and sewage sludge as compared to woody biomass is the lower weight percentage of lignin and the increased presence of cellulose and hemicelluloses, also fats in the case of sewage sludge. The lignin content of herbaceous resources typically ranges from 15% to a little over 20%.
Since lignin is less oxidized than hemicelluloses, it has a higher heating value and this typically translates to lower heating values of herbaceous biomass as compared to woody biomass or some agro-industrial residues, such as olive press cakes. The lower lignin content also affects, to some extent, the combustion speed.
Ultimate analysis – The elements
The elemental or ultimate analysis is the second typical way to present the components in the organic part of fuels. Instead of grouping compounds based on the chemical structure or the combustion behaviour, the ultimate analysis presents directly the main elements present in the organic part of biomass. In figure 1 above, the main elements (carbon, hydrogen and oxygen) are indicated while secondary elements like nitrogen, sulphur, chlorine and such are all grouped together in "other" since they typically amount to much less than the three major elements.
The ultimate analysis is also commonly referred to as the CHNS analysis on the basis of the most commonly measured elements; for herbaceous biomass, as for waste fractions, chlorine is also of major importance. The following key points can be made for each element:
Carbon (C) is obviously the most important constituent of biomass fuels. It mostly comes from the atmospheric CO2 that became part of the plant matter during photosynthesis. Carbon represents the major contribution to the overall heating value. During combustion, it is mainly transformed back into CO2, which is again released in the atmosphere.
In any combustion application, a part of the carbon is not combusted completely and leads to emissions of unburned gases, typically carbon monoxide or PAHs. However, this does not correlate to the overall carbon content but rather to combustion conditions and -equipment.
The carbon content of the fuel is directly related to its content of lignin, hemicellulose and cellulose. A low lignin content, like with herbaceous biomass, leads to a lower carbon content as compared to woody biomass. Typical values would be between 44 – 50% wt db. Fuels rich in lignin, such as olive kernel, have carbon contents higher than 50% wt db.
Hydrogen (H) is another major constituent of biomass, as can be expected from the chemical structure of the carbohydrate and phenolic polymers. During combustion, hydrogen is converted to H2O, significantly contributing to the overall heating value. As mentioned in Chapter 04-00, the hydrogen content affects the calculation of the lower heating value from the experimentally measured higher heating value. The weight content of hydrogen, on a dry basis, is usually slightly lower in herbaceous biomass (5.5 – 6 %) than in woody biomass, 6 – 8 %.
Nitrogen (N) is the most important nutrient for plants. It is absorbed via the soil or the applied N-fertilizers by the plant during its growth. Due to their high growth rate and the application of fertilizers, herbaceous biomass species have a higher N content (0.4 – 1.0 % wt db) compared to woody biomass types – even higher values have been reported for some grains. In some waste fractions, the content of nitrogen may be several %, a fact that contributes significantly to the degradability in biochemical processes like digestion or fermentation.
During combustion and for all practical purposes, nitrogen does not oxidize in any significant quantities and is released in the gas phase as N2 – therefore, its contribution to the overall heating value is zero. However, as will be discussed later on, what little nitrogen does get oxidized and converted to nitrogen oxides is an important gaseous emission from biomass systems. The high nitrogen content of herbaceous biomass may therefore result – but this is strongly depending on combustion conditions, -control and -equipment – in high emissions of nitrogen oxides.
Sulphur (S) is incorporated in several organic structures like amino-acids, proteins and enzymes. With waste fractions, where a mixture of organic substances forms the main part of the fuel, the content of sulphur may in some cases be significant. Along with nitrogen, phosphorus and potassium, it is an important nutrient for plant growth.
The high growth rate of most herbaceous crops means that the sulphur concentration in plant biomass is typically higher than those in woody biomass: while the concentration of sulphur in wood can be as low as 0% (which means below the detection limit of most laboratory devices) and reaches up to 0.1% on a dry basis in exceptional cases, herbaceous biomasses have a sulphur content ranging from 0 to 0.2% or even higher. In waste fractions, values up to approximately 1 % have been reported. Still, the sulphur content of these fuels is lower compared to most coals and certain types of liquid fossil fuels.
During combustion, sulphur is typically oxidized and has a minor contribution to the overall heating value. However, its most important impact relates to gaseous emissions (section 04-00-10), syngas cleaning in gasification processes (section 04-02-05) and corrosion issues (section 04-02-02a).
Chlorine (Cl) is the most important differentiation between herbaceous biomass and waste biomass on the one side and woody biomass or coals on the other side. While chlorine is typically found in negligible amounts in coals and in wood (< 0.05 % on a dry basis), herbaceous biomass species have a chlorine content ranging from less than 0.1% to 2% or more. Chlorine is absorbed via the plants through a variety of environmental sources and plays a role in certain plant functions. In waste fractions, the main part of the chlorine comes from salt present in food scrapings and from plastic (PVC) that has accidentally been sorted into the organic fraction.
During combustion, chlorine is almost completely vaporized, forming HCl, Cl2 and alkali chlorides. The problems associated with chlorine stem from issues related to emissions (dioxins, facilitation of aerosol formation) and operation issues, namely fouling and corrosion of metallic surfaces. Chlorine is not solely responsible for these issues. For fouling and corrosion, the effect of chlorine is greatly affected by the alkali content of the biomass as will be discussed in chapter 04-02. Generally though, chlorine content higher than 0.1% wt db is problematic.
Oxygen (O) is a major element in all biomass fuels, as is evident from the nature of the photosynthetic process and the chemical composition of the biomass constituents. Fuel oxygen reduces the amount of air needed for combustion and is found in the combustion products chemically bound in the molecules of CO2 and H2O. It should be noted that oxygen is not measured directly – its weight concentration is estimated by subtracting from 100 the concentrations of all other elements (C, H, N, S, Cl) and of the ash content in the dry fuel.
The measurement of the elemental composition for C, H and N is typically performed in laboratory equipment called elemental analyzers. The EN 15104 standard should be followed for their measurement. For sulphur and chlorine different procedures are described in the relevant standard, which is EN 15289. Typically though, sulphur can also be measured in an elemental analyzer, although according to the standard some validation with reference materials will be required.
Other methods to describe the fuel
Apart from the measurements described in the standards, there are obviously other means of characterizing a biomass fuel. A commonly used approach is the pyrolysis or combustion of a fuel in a thermo-gravimetric analyzer. By constantly monitoring the weight loss during each thermal process, the experimenter may obtain figures which provide useful indications for the thermal behaviour of biomass.
A typical example would be the pyrolysis of a biomass sample in an inert atmosphere under a constant heating rate (5 – 20 °C/min or more). By obtaining the weight loss curves, the total volatile content could be evaluated as well as the temperatures where the maximum rate of weight loss in observed. In addition, for most biomass samples, models for the simulation of the weight loss curves can be used in order to estimate the relative concentrations of cellulose, hemicelluloses and lignin as well as the kinetic parameters of their pyrolysis reactions.
Although not standardized (meaning that there may be significant differences in the approaches and results of different laboratories), such an analysis could prove useful to an engineer designing a combustion or gasification system for the investigated fuel.